Description:
The human ether-a-go-go related gene (hERG) encodes the inward rectifying voltage-gated
potassium channel in the heart (IKr) and is involved in cardiac repolarisation.
Inhibition of the hERG current causes QT interval prolongation, resulting in potentially
fatal ventricular tachyarrhythmia called Torsade de Pointes. Several drugs have
been withdrawn from late-stage clinical trials or had their use severely restricted
due to these cardiotoxic liability. Therefore, evaluating effects of compounds on
hERG activity early in drug discovery can significantly reduce the risk of putting
extensive efforts in cardiotoxic drugs. Additionally, most investigational new drug
(IND) applications require hERG channel pharmacological assessments to reduce the
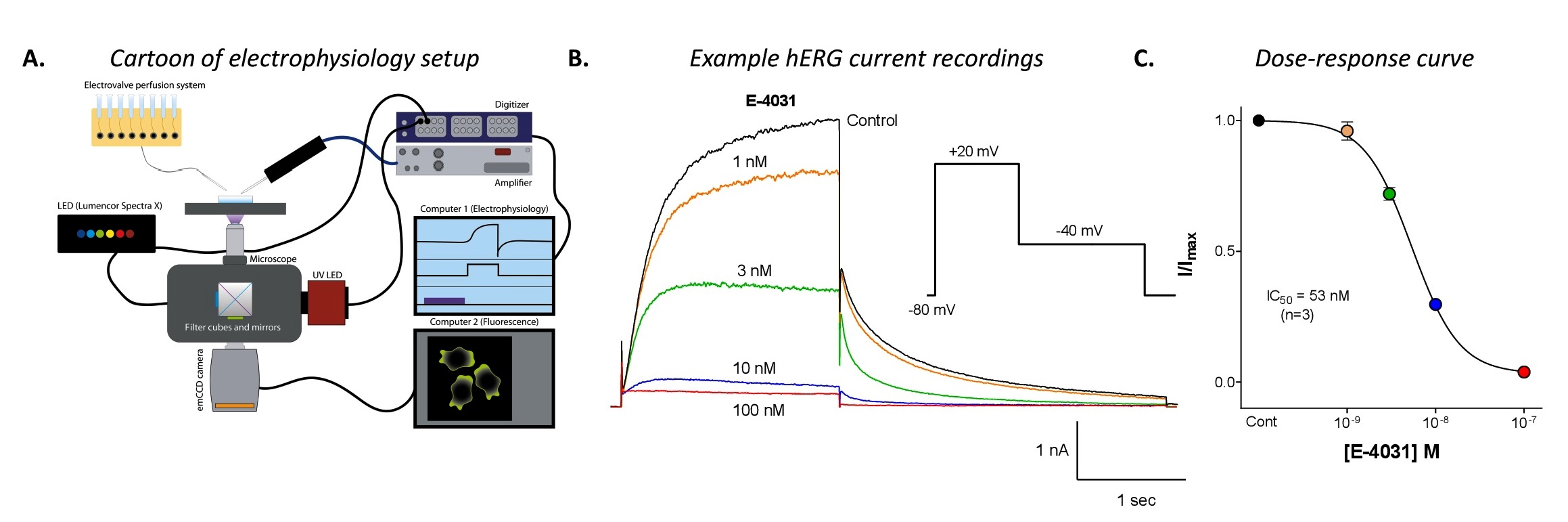
development of proarrhythmic drugs. CSIR-CDRI uses a conventional patch-clamping
electrophysiology system to assess the effect of test compounds on the hERG channels,
stably expressed in mammalian cells.
Technical Specifications:
HEK293-hERG cells that are in the exponential growth phase are used for compound
evaluation. Whole cell configuration of patch clamp technique will be used for recording
hERG channel currents in physiological solutions at room temperature. The voltage
stimulation protocol consists of a holding step at -80 mV to +20mV for 2 seconds
followed by a 3 second repolarization to -40mV (frequency: 0.1 Hz). The test compounds
are tested either at a single concentration or at 5 concentrations (cumulative concentration-response
ranging from 0.3-30 M). The percent change in hERG tail-current is calculated and
used to determine the IC50 value (test compound concentration that produces 50 %
inhibition).
Applications:
Approximately 60% of all new molecular entities aiming at cardiac or non-cardiac
targets interact with hERG channels; thus, these are considered “promiscuous targets”.
Thus, in vitro hERG toxicity assays have become integral component of regulatory
requirements. These preclinical studies help in predicting the likely risk/benefit
assessment in new drug application process.
Sample Requirements:
Test compound must be supplied as a dry powder (> 1mg) with its MSDS and CoA. In
addition, we will require the following information to be entered for each compound
on the Shipping Information Sheet:
- 1. Appropriate solubility of each test compound (e.g., DMSO, EtOH, H2O).
- 2. Concentrations required for testing.
- 3. Positive control request (e.g., E-4031).