Mycobacterium bovis sigF mutant exhibits altered surface phenotype and compromised pathogenesis
Sigma factor F (SigF) is an alternate sigma factor, widely conserved in pathogenic and non-pathogenic mycobacteria, suggesting its larger role in
addition to regulation of virulence genes. We generated a ΔsigF mutant in M. bovis which displayed distinct colony morphotype suggestive of
deficiency in surface properties (Fig 2A and 2B). The loss in phenotype was restored in the complemented ΔsigF mutant (Fig 2A and 2B). The ΔsigF
mutant showed marked depletion in key cord forming lipids, trehalose 6-6’-dimycolate, trehalose 6-monomycolate and phthiocerol dimycocerosate
(Fig 3B and 3E). Comparative proteomics revealed diminished level of several proteins predicted to have roles in cell surface properties, stress
response and virulence associated functions. Proteome analysis of M. bovis biofilms highlighted the role of SigF regulated proteins in biofilm
formation as several of them appeared at lower levels in the ΔsigF mutant. One of them was a key metabolic enzyme, malate synthase G (Mb1868c).
Consistent with its pleiotropic role, the diminished level of Mb1868c in the ΔsigF mutant resulted in reduced adherence of the mutant bacilli to
lung epithelial cells. In summary, we report novel morphotypes of the M. bovis ΔsigF mutant and provide rationale for their in vitro and in vivo
phenotypes, which improve our understanding of the role of SigF in mycobacterial biology.
Fig. Colony morphotypes of M. bovis wild-type (Mb), ΔsigF mutant (Mb10) and complemented strain (Mb11). (A) The ΔsigF mutant colonies appear smooth.
(B) Appearance of M. bovis strains on LJ slants. Accumulation of Nile red 2 µM (C) and EtBr 6 µM (D) by M. bovis wild-type (Mb), ∆sigF mutant (Mb10)
and complemented strain (Mb11) were measured by fluorescence spectroscopy. The data are mean ± SD from three independent experiments
(**** p <0.0001).
Fig. 2D-TLC analysis. Nonpolar lipids from M. bovis wild-type (A), ΔsigF mutant (B) and complemented strain (C). Polar lipids from M. bovis wild-type
(D), ΔsigF mutant (E) and complemented strain (F). The arrows indicate the differential lipids content. PDIM, TMM and TDM refer to Phthiocerol
dimycocerosate, Trehalose monomycolate and Trehalose dimycolate, respectively. (G) Shows the quantitation of the PDIM levels in different strains
using image analysis software (GE Healthcare). The data were normalized to those for the wild-type strain and represent mean ± SD of three
independent experiments (*** p <0.001). (H) DNase treatment of M. bovis biofilms. M. bovis wild-type biofilms were incubated with PBS, PBS and
buffer (reaction buffer), PBS + DNase, heat inactivated (HI) DNase and buffer + DNase. Biofilms were quantified via crystal violet staining. Data
represent mean ± SD of three independent experiments (*** p <0.001).
Development of novel antimycobacterial peptides using database filtering and three-dimensional modeling approach
The cell wall of Mycobacterium tuberculosis (Mtb) provides a major defensive mechanism from antibiotics, phagocytic vesicles in macrophages and d
esiccation in dry sputum. Host defense peptides (HDPs), our "natural antibiotics", are major component of innate immunity and evolutionary
conserved, host defense molecules, having a direct action on microbial membranes with a low risk of resistance development. The peptides which
have potential to assume helical structure in liquid cultures do have antimicrobial activity. Also, the moderately short cationic and hydrophobic
peptides derived from the conserved domains of various proteins have antimicrobial activity under physiological conditions without any toxicity
issues. However, the composition of the mycobacterial membrane differs significantly from that of bacteria, and it cannot be concluded that all
AMPs, which have been identified as pore/ lesion-forming HDPs against bacteria, also induce lesions in mycobacteria. We predicted that the helical
domains of Glutathione s-transferase, (GST)-theta might have antimycobacterial activity or could give us templates for further optimization. So in
this work, we designed potent HDPs than those found in nature, to construct a random in silico peptide library. Then by using “database filtering”
and three-dimensional (3D) modeling approach peptide sequences were selected for the synthesis, followed by in vitro analysis. We explored peptides
derived from the GST for antimycobacterial activity. The candidate peptides, shown in wheel diagram representations (Fig 4), were developed. The
helical wheel diagrams demonstrate the amphipathic nature of peptides and in order to see the effect of peptides on membranes, we did a scanning
electron microscopy study with peptides at 2X MIC concentrations (Fig. 5).
The results show that all the tested peptides induced cytoplasmic condensation and cell wall modification (thinning and budding) (YRA-15 and YWW-15)
or destruction (RWW-15 and YQA-15). The cytotoxicity studies indicated that the peptides were not toxic to the human cells. In the optimized series,
peptide with higher charge but lower hydrophobicity, showed signs of toxicity at the lowest concentration (50 µM), whereas the peptides with lower
charge and higher hydrophobicity showed lower toxicity (Fig 5). Among all the peptides four peptides showed significant gain in the activity. This
study indicates that peptide to be active against Mycobacterium tuberculosis H37Ra should have a charge of +4 and hydrophobicity > 60% and less
than 70%.
Fig. Scanning electron microscopy images showing the mode of action of the peptides (upper) and cytotoxicity profile (lower) of hit molecules.
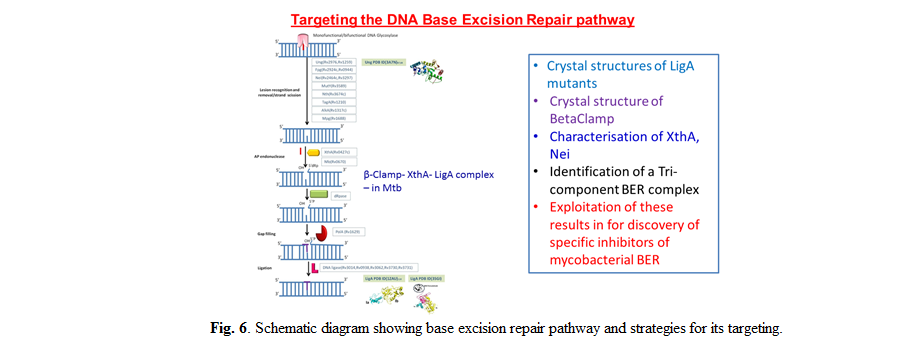
Identification of fragment inhibitors of Mycobacterium tuberculosis NAD+-dependent DNA Ligase A that target ‘serial-remodeling’
The NAD+-dependent DNA ligase (LigA) is a multi-domain, essential, bacterial enzyme that functions as the principal replicative ligase. Differences
between the human ATP-dependent DNA Ligase-I (Lig1) and LigA makes the latter an attractive antibacterial target. We solved co-crystal structures with
NMN, AMP and inhibitors identified from fragment-screening. Inhibitors reported previously mainly compete with AMP and bind to the 1B sub-domain of
the adenylation domain. We recently screened a fragment library against the NMN-binding pocket and identified 2 fragments that inhibit LigA activity
with IC50 in the low µM range. Experiments involving E. coli GR501 strain that harbors its own temperature-sensitive LigA, rescued with Mtb LigA,
show that the fragments inhibit bacterial growth by targeting Mtb LigA in vivo. Additionally, the experiments showed that the inhibitors can
distinguish between NAD+- and ATP-dependent ligases. The NMN binding pocket on subdomain 1A has been shown to be a druggable target site for
developing anti-LigA therapeutic strategies.
Nef regulates protein trafficking in HIV Pathogenesis
HIV-1 Nef regulates several cellular functions in an infected cell. The molecular mechanism(s) underlying Nef-dependent cellular function(s) are
unable to define how the events are coordinately regulated in the host cell. In this study, our laboratory deciphered the role of Nef on Rab
GTPases - dependent complex vesicular trafficking. Expression profiling of Rabs in Nef-expressing cells showed that Nef differentially regulates
the expression of individual Rabs in a cell-specific manner. Further analysis of Rabs in HIV-1NL4-3 or ΔNef infected cells demonstrated that Nef
protein was responsible for variation in Rabs expression. Using a panel of competitive peptide inhibitors against Nef, we identified the critical
domain of HIV-1 Nef involved in modulation of Rabs expression. The molecular function of Nef-mediated up-regulation of Rab5 and Rab7 and
down-regulation of Rab11 increased the transport of SERINC5 from the cell surface to lysosomal compartment. Moreover, Nef-dependent increase in
Rab27 expression assists its own release via exosomes. Reversal of Rabs expression using competitive inhibitors against Nef reduced viral release
and infectivity of progeny virions. Overall, this study uncovers a new paradigm that Nef differentially regulate the expression of Rabs protein in
infected cells to hijack the host intracellular trafficking, which augment viral replication and HIV-1 pathogenesis.
A long-term lipid only diet can convert Mycobacterium tuberculosis to hard-to-grow persisters
The differentially detectable (DD) persisters, of Mycobacterium tuberculosis (Mtb), are persisters that fail to form colonies on agar media when
de-stressed. Since in the host, Mtb primarily survives by utilizing lipids, we used a long-term lipid diet model to induce DD persisters of Mtb.
Persisters were induced by replacing the dextrose-containing medium with one containing fatty acids (FAM). After 2, 4 or 6 weeks, CFU and most
probable number assays were performed; the difference between the two gave an estimate of DD persisters (Fig. 7). Since rifampicin has been shown
to induce formation of DD persisters in vitro, one set of FAM cultures were also given short- term rifampicin stress after 2, 4 or 6 weeks.
Fig. The fraction of DD persisters increased with time and rifampicin treatment enhanced the effect of fatty acids, at 2 and 4 weeks. At six weeks,
even in the absence of rifampicin, approximately 95 % cells were DD persisters. The DD persisters (Fig. panel B) were found to be more drug tolerant
than the colony forming persisters (Fig. panel A) under the similar culture conditions. The DD persisters were vulnerable to drugs interfering with
bacterial respiration such as thioridazine (TRZ), bedaquiline and clofazimine (Figure), but were tolerant to rifampicin and moxifloxacin. The study
indicates potential formation of DD persisters of Mtb in a lipid-rich microenvironment in the host even before antibiotic therapy.