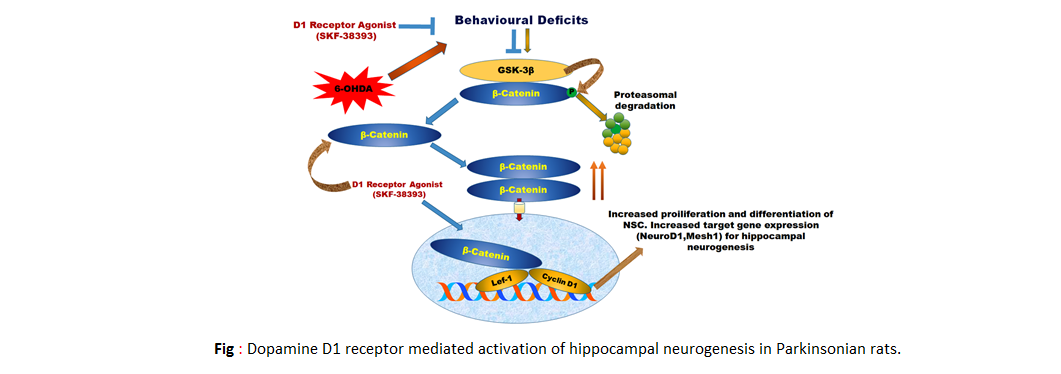
Dopamine D1 receptor activation improves adult hippocampal neurogenesis and exerts anxiolytic and antidepressant-like effect in rat model
of Parkinson's disease/span>
Parkinson’s disease (PD) is primarily characterized by midbrain dopamine depletion. Dopamine acts through dopamine receptors (D1 to D5) to regulate locomotion, motivation, pleasure, attention,
cognitive functions and formation of newborn neurons, all of which are likely to be impaired in PD. Reduced hippocampal neurogenesis associated with dopamine depletion
has been demonstrated in patients with PD. However, the precise mechanism to regulate multiple steps of adult hippocampal neurogenesis by dopamine receptor(s) is still
unknown. This study tested whether pharmacological agonism and antagonism of dopamine D1 and D2 receptor regulate nonmotor symptoms, neural stem cell (NSC) proliferation
and fate specification and explored the cellular mechanism(s) underlying dopamine receptor (D1 and D2)- mediated adult hippocampal neurogenesis in rat model of PD-like
phenotypes. It was found that single unilateral intra-medial forebrain bundle administration of 6-hydroxydopamine (6-OHDA) reduced D1 receptor level in the hippocampus.
Pharmacological agonism of D1 receptor exerts anxiolytic and antidepressant-like effects as well as enhanced NSC proliferation, long-term survival and neuronal
differentiation by positively regulating Wnt/β-catenin signaling pathway in hippocampus in PD rats. shRNA lentivirus mediated knockdown of Axin-2, a negative
regulator of Wnt/β-catenin signaling potentially attenuated D1 receptor antagonist induced anxiety and depression-like phenotypes and impairment in adult hippocampal
neurogenesis in PD rats. These results suggest that improved nonmotor symptoms and hippocampal neurogenesis in PD rats is controlled by D1-like receptors and involve
the activation of Wnt/β-catenin signaling (Neurochem Int. 2019; Jan; 122:170-186).
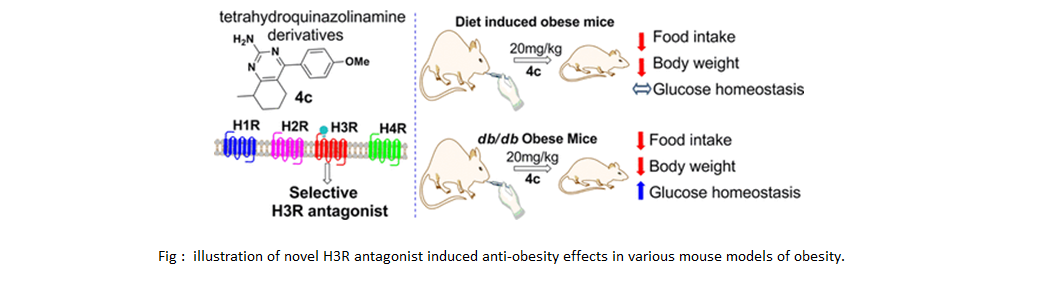
Novel Tetrahydroquinazolinamines as selective Histamine 3 receptor antagonists for the treatment of obesity
The histamine 3 receptor (H3R) is a presynaptic receptor, which modulates several neurotransmitters including
histamine and various essential physiological processes, such as feeding, arousal, cognition, and pain. The H3R is considered as a drug
target for the treatment of several central nervous system disorders. In this study, synthesized and identified a novel series of
4-aryl-6-methyl-5,6,7,8-tetrahydroquinazolinamines that act as selective H3R antagonists. Among all the synthesized compounds, in vitro
and docking studies suggested that the 4-methoxy-phenyl-substituted tetrahydroquinazolinamine compound 4c has potent and selective H3R
antagonist activity (IC50 < 0.04 μM). Compound 4c did not exhibit any activity on the hERG ion channel and pan-assay interference compounds
liability. Pharmacokinetic studies showed that 4c crosses the blood brain barrier, and in vivo studies demonstrated that 4c induces anorexia
and weight loss in obese, but not in lean mice. These data reveal the therapeutic potential of 4c as an anti-obesity candidate drug via
antagonizing the H3R.