Summary of drug discovery and development activities
During the reporting period several compounds were synthesised and evaluated for cardiovascular activities in cell culture and in animal models. Around 87, 73, 80, 80,
216 compounds were submitted for Anti-Inflammatory, Anti-hyperlipidemic, Anti-Angiogenic, Anti-NAFLD and Anti-adipogenesis activities,
respectively. 12, 8 and 2 interesting hits were obtained in the anti-hyperlipidemia, anti-angiogenic and anti-adipogenic screens respectively
which are being further validated in the respective laboratories. Furthermore, about 600 NCEs and natural products were submitted for GPCR
profiling during last one year and several preliminary hits were identified that are being consolidated. Besides these, several standardized
extracts like CDR267F018 were evaluated for cardiovascular activities under CSIR’s Phytomission.
New initiatives in the areas of CVS disorders
Several new dimensions were added to the area of cardiovascular research with the joining of new faculty in the
Pharmacology department. A new area for the identification of novel theranostic targets in heart diseases was initiated. Cardiovascular
diseases are the leading cause of mortality in India as well as globally. According to the recent data from Global Burden of Diseases, in
India, cardiovascular diseases contributed to more than twenty-six percent of deaths in 2017. Heart failure characterized by inability of
the heart to pump enough blood to the body, is the end-result of various pathological events collectively termed as cardiac remodelling.
Morphologically, cardiac remodelling involves cardiomyocyte hypertrophy/atrophy, apoptosis, deregulated autophagy and interstitial fibrosis.
Therefore, it is hypothesized that better understanding of cardiac remodelling process will help in identification of novel theranostic
target, which might reduce cardiovascular disease burden. New studies are initiated on the exploration of novel RNA-binding proteins and
circular RNAs as theranostic target. Workers aim to utilize viral gene therapy approach like adeno-associated virus to inhibit and increase
the expression of these genes in diseased heart.
Augmentation of iNOS expression in myeloid progenitor cells expedites neutrophil differentiation
Neutrophils play an important role in immunity and inflammation through diverse mechanisms. Previously involvement of NO in neutrophil adhesion,
chemotaxis, bacterial killing, reactive oxygen species generation, neutrophil extracellular traps' formation, and apoptosis has been demonstrated.
Constitutive expression of iNOS in human neutrophils has also been documented. The role of NO-iNOS in neutrophil differentiation however remains
ill-defined. Herein, studied the role of NO generated from iNOS in the neutrophil differentiation by using iNOS-overexpressing K562 cells (K562iNOS)
and iNOS-deficient murine progenitor cells (lineage negative cells; lin-ve). It was observed that iNOS overexpression led to increased neutrophilic
differentiation in K562 cells; more specifically an early and accelerated neutrophilic differentiation was spotted in K562iNOS. These observations
were further validated using iNOS knockout lin-ve cells or hematopoietic progenitor cells that exhibited delayed neutrophil differentiation in
comparison to its wild-type counterpart. In addition, a significant increase in the gene expression of iNOS during neutrophilic differentiation of
CD34+ hematopoietic stem and progenitor cells derived from human bone marrow further substantiates importance of iNOS in neutrophil differentiation.
Moreover, a significant increase in NO generation during neutrophil differentiation was observed and enhanced neutrophil differentiation with NO
donor was also observed, implying the importance of NO in neutrophil differentiation. Collectively, using alternative approaches, it is
demonstrated that neutrophil differentiation is significantly influenced by iNOS or NO, suggesting the possibility of exploiting this novel link
for therapeutic aspects of NO generated from iNOS and neutrophil differentiation in hematopoiesis-related disorders. (J Leukoc Biol. 2019; 106(2):
397-412).
Role of P47phox in Angiotensin II induced cardiac hypertrophy and fibrosis in mice
NADPH oxidase mediated ROS production has been recognized to be an important contributory mechanism for hypertension and left ventricular hypertrophy
(LVH). However, the regulatory role of NADPH oxidase mediated superoxide production in Angiotensin II (AngII) induced cardiac fibrosis and
macrophage infiltration is still elusive. Herein, investigated the role of phagocytic NADPH oxidase enzyme system in cardiac remodelling
induced by AngII in young adult (2-3 months old) male mice deficient in p47phox, a cytosolic subunit of the NADPH oxidase (KO, n=13) and
age-matched wild-type littermates (WT, n=9). Animals were infused with AngII pressor dose (1500 ng/kg/min, s.c) or saline via osmotic
mini pump for 14 consecutive days and subjected to echocardiography on the last day. Further animals were sacrificed and hearts were
harvested for histological studies and gene expression analysis. AngII treatment in WT and KO mice showed prominent alterations in
echocardiographic measurements with no changes in E/A, EF and FS. However, KO mice showed aggravated cardiac hypertrophic response
(LVAWd: WT, 1.20±0.09 vs. KO, 1.42±0.04, p<0.05; LVIDd: WT, 3.02±0.20 vs. KO, 2.60±0.07, p<0.05; LVPWd: WT, 1.49±0.06 vs. KO, 1.78±0.06, p<0.05).
Histologically, AngII infused KO mice also showed increased cardiomyocyte diameter compared to AngII infused WT mice
(WT, 388±16.37 vs. KO, 560.3±41.28 μm2; p<0.01 respectively). Further, AngII infused KO mice showed elevated immune cell infiltration, which
were positive for Mac-3 staining compared to AngII infused WT mice (WT, 2.61±0.32 vs. KO, 4.09±0.44 %; p<0.05). Besides, AngII infused KO mice
showed augmented interstitial fibrosis (WT, 11.11±0 0.67 vs. KO, 20.85± 3.63 %; p<0.05), thick collagen fibres deposition
(WT, 28.93± 0.94 vs. KO, 37.60± 2.49 %; p<0.05) compared to AngII infused WT mice. Moreover, AngII infused KO mice also showed upregulated
gene expression of hypertrophic and fibrotic markers, including Nppa, Nppb, Acta1, Myh6, TGF-β1, collagen (I and III) and α-sma compared to
AngII infused WT mice. Together, these data suggest that NADPH oxidase and its subunit p47phox play a pivotal role in AngII induced LVH by
regulating fibrotic machinery and macrophage infiltration (Hypertension, 2019; Vol. 74, No. Suppl-1).
Temporal immmunometabolic profiling of adipose tissue in HFD-induced obesity: Manifestations of mast cells in fibrosis and senescence
Chronic low-grade inflammation/meta-inflammation in adipose tissue leads to obesity-associated metabolic complications. Despite growing understanding,
the roles of immune cell subsets, their interrelationship and chronological events leading to progression of obesity-associated insulin
resistance (IR) remains unclear. Herein, carried out temporal immunometabolic profiling of adipose tissue from C57BL/6 mice fed a high-fat
diet (HFD) for 4, 8, 12, 16, and 20 weeks. Clodronate sodium liposomes (CLODs) were used to deplete macrophages and disodium cromoglycate
sodium liposomes (DSCGs) to stabilize mast cells. In the temporal HFD settings, mice showed progressive glucose intolerance, insulin
resistance, and adipose tissue senescence. Histochemical analysis of epididymal white adipose tissue (eWAT) using picro-sirius red and
Masson's trichrome staining showed extensive collagen deposition in the 16th and 20th weeks. Flow cytometry analysis of the stromal
vascular fraction (SVF) from eWAT revealed T-cell subsets as early-phase components and pro-inflammatory macrophages, as well as mast
cells as the later phase components during obesity progression. In this therapeutic strategy, macrophage depletion by CLOD and mast
stabilization by DSCG attenuated obesity, adipose tissue fibrosis, and improved whole-body glucose homeostasis. In addition, mast cell
stabilization also attenuated senescence (p53 and X-gal staining) in eWAT, signifying the role of mast cells over macrophages during
obesity. The study emphasizes on the new-generation mast cell stabilizers that can be exploited for the treatment of obesity-associated
metabolic complications (Int J Obes (Lond). 2019; 43(6): 1281-1294).
Pro-inflammatory effect of EMP is dysfunctional mitochondria mediated and modulated through MAPKAPK2 leading to attenuation of cardiac hypertrophy
This Study showed the functional significance of mitochondria present in EMP and how MK2 (MAPKAPK2) governs EMP production and its
physiological effect on cardiac hypertrophy. Flow cytometric analysis, confocal imaging, OCR measurement through Seahorse were used to
confirm the presence of functionally active mitochondria in non-treated EMP (c-EMP), LPS and oligomycin treatment increased mitochondrial
ROS activity in EMP (l- and o-EMP, respectively). The dysfunctional mitochondria contained in l- and o-EMP induced the expression of
pro-inflammatory mediators in the target endothelial cells leading to the augmented adhesion of THP-1 monocyte cells on EA.hy926 cells.
Multiphoton real-time imaging detected the increased adherence of o-EMP at the site of carotid artery injury as compared to c-EMP.
MK2-deficient EMP reduced the E-selectin and ICAM-1 expression on target endothelial cells leading to reduced monocyte attachment and
reduced cardiac hypertrophy in mice. In conclusion MK2 promotes the pro-inflammatory effect of EMP mediated through dysfunctional
mitochondria. MK2 modulates the inflammatory effect induced during cardiac hypertrophy through EMP. (Arterioscler Thromb Vasc Biol.
2019 Jun; 39(6): 1100-1112).
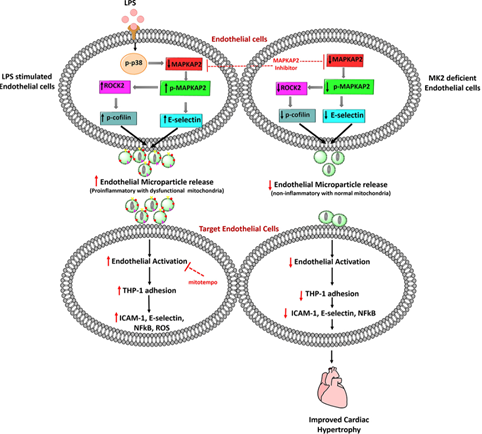
Fig: Cartoon depicting how dysfunctional mitochondria loaded EMP regulates pro-inflammatory mediators via MAPKAPK2
Team Members:

Above Left to Right : Dr Shashi Kumar Gupta, Dr Baisakhi Moharana, Dr Kashif Hanif, Dr Rajendra Singh, Dr Sachin Kumar, Dr Jayanta Sarkar, Dr Amit Lahiri, Dr Aamir Nazir, Dr Rajesh Jha
Below Left to Right : Dr Monika Sachdeva, Dr Manoj Kumar Barthwal, Dr Anila Dwivedi, Dr Gopal Gupta, Dr P.N. Yadav, Dr W Haq, Dr Anil Nilkanth Gaikwad, Dr S.K. Rath, Dr Sarika Singh, Dr Smrati Bhadauria