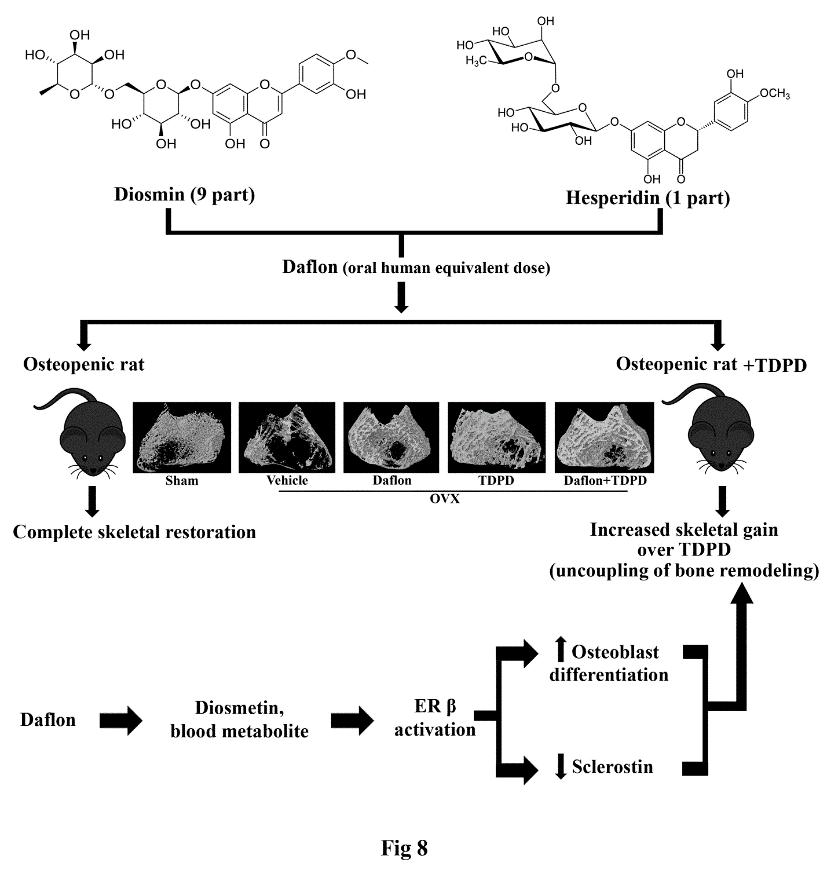
1.1.7.2 Daflon has osteogenic and anti-resorptive effects and enhances the skeletal effect of teriparatide: evidence of estrogen receptor in mediating skeletal effects of the drug
Flavanone glycoside containing drug, daflonR (diosmin/hesperidin, 9:1 combination) is in clinical use for the treatment of chronic venous insufficiency for many
years. Here, we showed that at the human equivalent dose daflon promoted bone regeneration at the osteotomy site and peak bone accrual in rat models. Daflon
restored trabecular bones and strength with attendant increases in surface referent bone formation parameters and serum osteogenic marker in osteopenic OVX
rats. Daflon also suppressed bone resorption in OVX rats and OVX rats treated with teriparatide [human parathyroid hormone (PTH 1-34), TDPD] without inhibiting
the osteoanabolic effect of TDPD. Moreover, the combination of daflon and TDPD showed greater skeletal effect than the monotherapies. PK studies revealed that
oral administration of daflon resulted in the formation of diosmetin, the aglycone form of diosmin. Subsequent studies showed that diosmetin specifically
activated estrogen receptor- (ER) resulting in the stimulation of osteoblast differentiation and suppressed the anti-osteoblastogenic factor, sclerostin.
Our findings of osteoanabolic effect of daflon accompanied by its ability to enhance the skeletal effect of TDPD could lead to a new class for anti-osteoporosis
therapy through therapeutic repurposing (Biomed PharmacotherPMID: 31306971)
1.1.7.3 The osteogenic effect of liraglutide involves enhanced mitochondrial biogenesis in osteoblasts
Liraglutide (Lira), a long-acting glucagon-like peptide 1 receptor (GLP-1R) agonist reduces glycosylated hemoglobin in type 2 diabetes mellitus patients. Here,
we investigated the osteoanabolic effect of Lira and studied the underlying mechanism. In established osteopenic OVX rats, Lira completely restored bone mass
and strength comparable to parathyroid hormone (PTH). The serum levels of osteogenic surrogate pro-collagen type 1 N-terminal pro-peptide (P1NP) and surface
referent bone formation parameters were comparable between Lira and PTH. GLP-1R, adiponectin receptor 1 (AdipoR1) and peroxisome proliferator-activated receptor
gamma coactivator 1-alpha (PGC1) levels in bones were downregulated in the OVX group but restored in the Lira group whereas PTH had no effect. In cultured
osteoblasts, Lira time-dependently increased GLP-1R, AdipoR1 and PGC-1. In osteoblasts, Lira rapidly phosphorylated AMP-dependent protein kinase (AMPK), the
cellular energy sensor. Exendin 3, a selective GLP-1R antagonist and PKA inhibitor H89 blocked Lira-induced increases in osteoblast differentiation, and
expression levels of AdipoR1 and PGC-1. Lira increased mitochondrial number, respiratory proteins and respiration in osteoblasts in vitro and in vivo,
and blocking mitochondrial respiration mitigated Lira-induced osteoblast differentiation. Taken together, our data show that Lira has a strong osteoanabolic
effect which involves upregulation of mitochondrial function.(BiochemPharmacolPMID: 30885766)
2.2 Progress on advancing in knowledge frontiers
2.2.1 MicroRNA-672-5p identified during weaning reverses osteopenia and sarcopenia in ovariectomized mice
Post-menopausal condition augments the biological aging process, characterized by multiple metabolic disorders in which bone loss is the most prevalent outcome
and usually coupled with sarcopenia. Coexistence of such associated pathogenesis have much worse health outcomes, compared to individuals with osteoporosis
only. Pre- and post-natal bone development demands calcium from mother to fetus during pregnancy and lactation leading to a significant maternal skeletal loss.
It follows an anabolic phase around weaning during which there is a notable recovery of the maternal skeleton. Here, we have studied the therapeutic effect of
microRNA-672-5p identified during weaning when it is predominantly expressed, in ovariectomized mice for both osteopenia and sarcopenia. miR-672-5p induced
osteoblast differentiation and mineralization. These actions were mediated through inhibition of Smurf1 with enhanced Runx2 transcriptional activation. In vivo,
miR-672-5p significantly increased osteoblastogenesis and mineralization, thus reversing bone loss caused by ovariectomy. It also improved bone-mineral density,
load-bearing capacity, and bone quality. Sarcopenia was also alleviated by miR-672-5p, as we observed increased cross-sectional area and Feret’s diameter of
muscle fibers. We hypothesize that elevated miR-672-5p expression has therapeutic efficacy in estrogen-deficiency-induced osteopenia along with sarcopenia.
(MolTher Nucleic Acids PMID: 30769134)
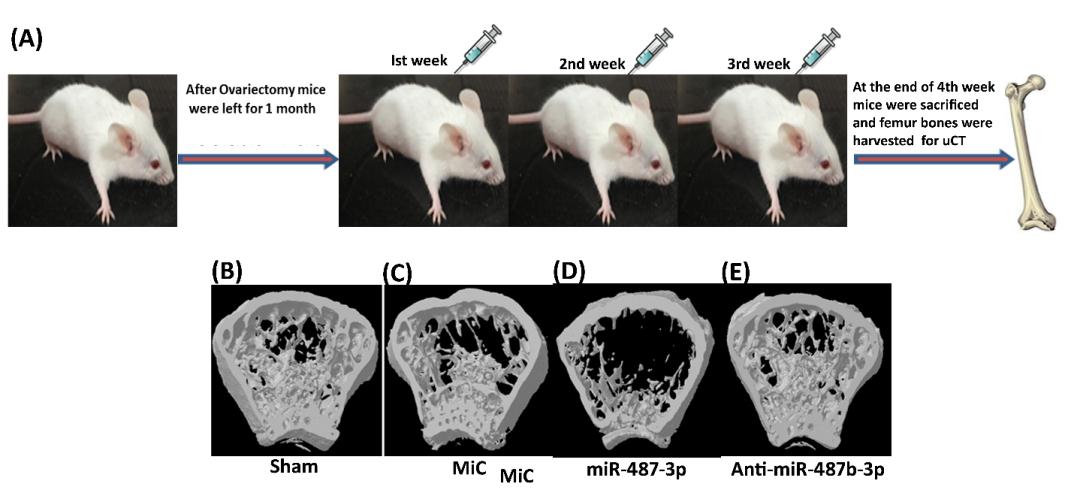
2.2.2 MiR-487b-3p impairs osteoblastogenesis by targeting Notch-regulated ankyrin-repeat protein
The study reports the role of miR-487b-3p in
regulation of osteoblast functions. Over-expression of miR-487b-3p leads to inhibition of osteoblastic differentiation. Using in silico approaches, Nrarp was
found to be the direct target of miR-487b-3p which was further validated by luciferase 3′ UTR reporter assay. Nrarp inhibits Notch-1 signaling and promotes
Wnt signaling by stabilization of LEF-1. Protein levels of Nrarp, RUNX-2, Lef1 and β catenin were reduced in osteoblasts cells transfected with miR-487b-3p
whereas protein levels of Notch1, Hes1 and P- β catenin were up regulated when osteoblast cells were transfected with miR-487b-3p. These outcomes were reversed
after treating cells with anti-miR-487b-3p. Further silencing of miR-487b-3p in neonatal and Ovx Balb/c mice attenuated all the inhibitory actions of miR-487b-3p
on osteoblast differentiation. Overall, miR-487b-3p negatively regulates osteogenesis by suppressing Nrarp expression, which in turn, suppresses Runx-2 and
Wntsignalling, both of which play a pivotal action in osteoblast differentiation. (J Endocrinol. 2019;241(3):249-263.).
2.2.3 Increased bone marrow-specific adipogenesis by clofazimine causes impaired fracture healing, osteopenia and osteonecrosis without extra-skeletal effects in rats
Mycobacterium leprae infection causes bone lesions and osteoporosis, however, the effect of anti-leprosy drugs on the bone is unknown. We, therefore, set out to
address it by investigating osteogenic differentiation from bone marrow derived mesenchyme stem cells. Out of seven anti-leprosy drugs, only clofazimine (CFZ)
reduced MSCs viability (IC50 ~1M) and their osteogenic differentiation but increased adipogenic differentiation on a par with rosiglitazone, and this effect was
blocked by a peroxisome proliferator-activated receptor gamma(PPARγ) antagonist, GW9662. CFZ also decreased osteoblast viability and resulted in impaired bone
regeneration in a rat femur osteotomy model at 1/3rd human drug dose owing to increased callus adipogenesis as GW9662 prevented this effect. In adult rats, CFZ
caused osteopenia in long bones marked by suppressed osteoblast function due to enhanced adipogenesis and increased osteoclast functions. A robust increase in
marrow adipose tissue (MAT) by CFZ did not alter hematologic parameters but likely reduced BM vascular bed leading to osteonecrosis (ON) characterized by empty
osteocyte lacunae. From these data, we conclude that CFZ has skeletal toxicity and could be used for creating a rodent ON model devoid of extra-skeletal effects.
(ToxicolSciPMID: 31393584)
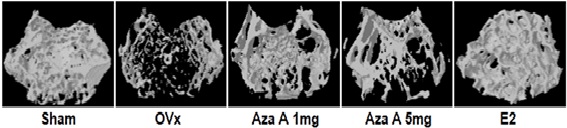
2.2.4 Estrogen receptor activation in response to Azadirachtin A stimulates osteoblast differentiation and bone formation in mice
In this study, it was seen that a natural pure compound Azadirachtin A (Aza A) isolated from Azadirachtaindica binds selectively to a site in the estrogen
receptor, identifying itself to be a selective tissue modifier. Using computational and medicinal chemistry, Aza A was shown to bind electively to estrogen
receptor‐α (ERα) as compared with ERβ. This preferential binding of Aza A to ERα with good pharmacokinetic distribution in the body forms metabolites, showing
that it is well absorbed. In in vivo estrogen deficiency models for osteoporosis, Aza A at a much lower dose enhances new bone formation at both sites of the
trabecular and cortical bone with increased bone strength and presents with no hyperplastic effect in the uterus. (J Cell PhysiolPMID:31225646)