Ageing is a critical risk factor for a variety of human pathologies. In order to meet the ever-growing healthcare demands of ageing human populations, it is pertinent to delineate the cellular and molecular processes that change with age and lead to increased disease susceptibility and frailty. A decline in mitochondrial quality and activity is associated with normal ageing and correlates with the development of many age-related diseases. Interestingly, the mitochondrial function is not only defined by the status of metabolic / nutrient signalling pathways, but also regulated post-transcriptionally by small RNAs, such as miRNAs. Recently, in addition to the cytosol, a fraction of miRNAs have been identified within the mitochondrial matrix itself.
While miRNAs have been studied for years, the function of (mitochondrial-localized miRNAs) mitomiRs and their role in ageing and age-associated pathologies remains largely unexplored. These mitomiRs are both nuclear and mitochondrial in origin and act both in and/or out of the mitochondria. Discrete populations of mitomiRs have been identified in mitochondria of different species and cell types. These findings suggest that the functional relationship between mitochondria and mitomiRs is possibly cell type-specific. With ageing, cellular metabolism and mitochondrial homeostasis constantly changes, thus we hypothesize that the population of mitomiRs also varies with age and contribute to these cellular and molecular changes. Therefore, the main aim of our lab is to understand the role of mitomiRs in ageing and age-associated Neurological Disorders.
We are interested in understanding the mitomiRs dynamics over the course of natural ageing using Caenorhabditis elegans as the model system. More specifically, we aim to elucidate the possible effects of mitomiRs on ageing and age associated pathologies along with their underlying molecular and functional mechanisms; tissue specificity and evolutionary relevance. Age-associated novel mitomiRs and their targets will be identified using deep-Sequencing of small RNAs, big data analysis, target prediction, and fluorescence microscopy. This information will further be utilized to develop genetic and pharmacological interventions.
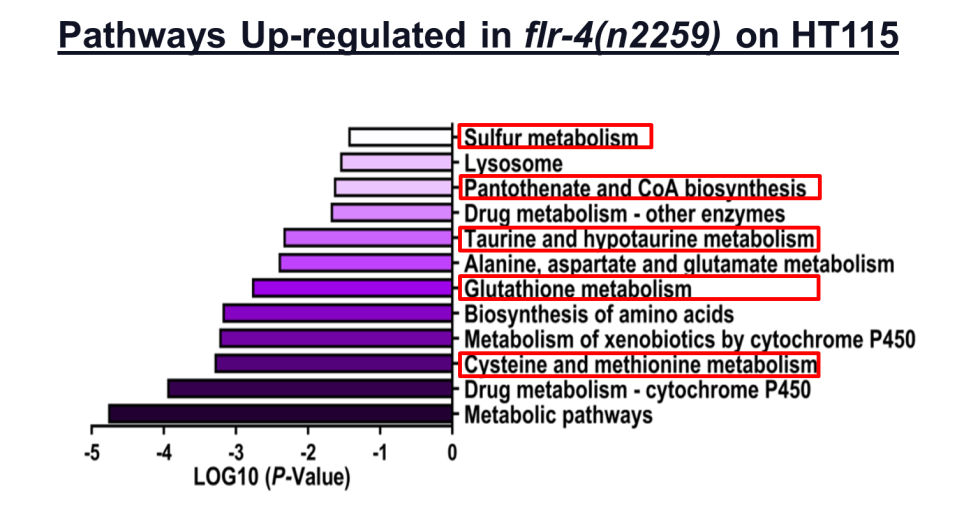
Up-regulation of pathways involved in maintenance of mitochondrial homeostasis (in red box) to promote longevity. (Verma et al. 2018).
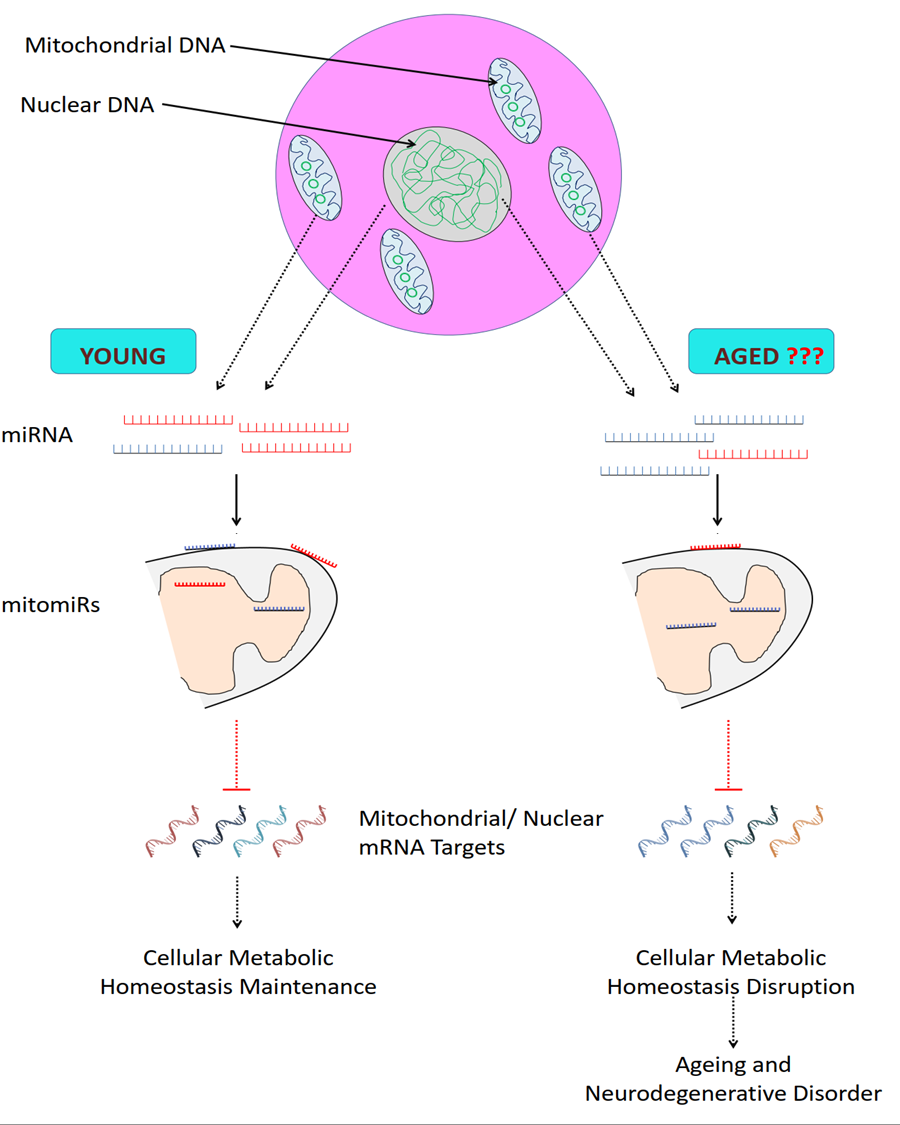
Graphical abstract for mitomRs mediated longevity.The population of mitomiRs and their targets may vary between young and aged cell, eventually modulating cellular metabolic status and thus ageing.