Introduction
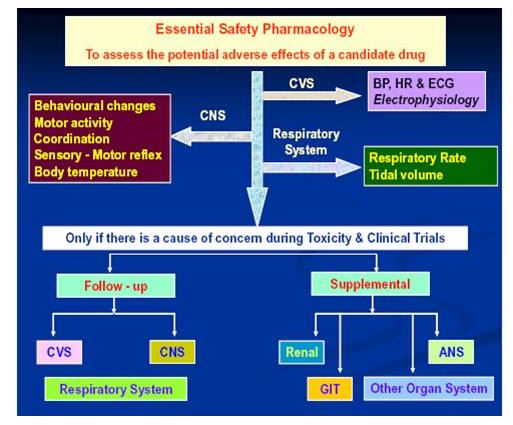
Regulatory pharmacological studies constitute an essential part of pre-clinical investigations and are designed to find out whether a substance has potential pharmacological activity in organ systems other than the one targeted for therapeutic purposes. It is desirable that a candidate drug to be developed should be devoid of any significant effect on other systems. These studies intend to collect information on the occurrence of potential adverse effects. The regulatory pharmacology programme consists of the following test systems in experimental animals, usually mice and rats, in the following areas: General behavioral study, acute toxicity determination, CNS, Skeletal muscle, cardiac system, Respiration Gastrointestinal tract and renal function.
Team Members
Team of Coordinators
- Dr. Manoj K. Barthwal
- Dr. Anil Gaikwad
- Dr. Prem N Yadav
- Dr. Kumaravelu Jagavelu
- Dr. Kashif Hanif
- Dr. Shubha Shukla
- Dr. Baisakhi Moharana