Our specific research goals includes:
1) Development of novel therapeutics against Dengue and JEV menace
2) Development of safe and cost-effective subunit vaccine candidates against JEV
3) Investigate the biomarkers and immune responses involve in dengue disease severity in terms of co-infection with related viruses
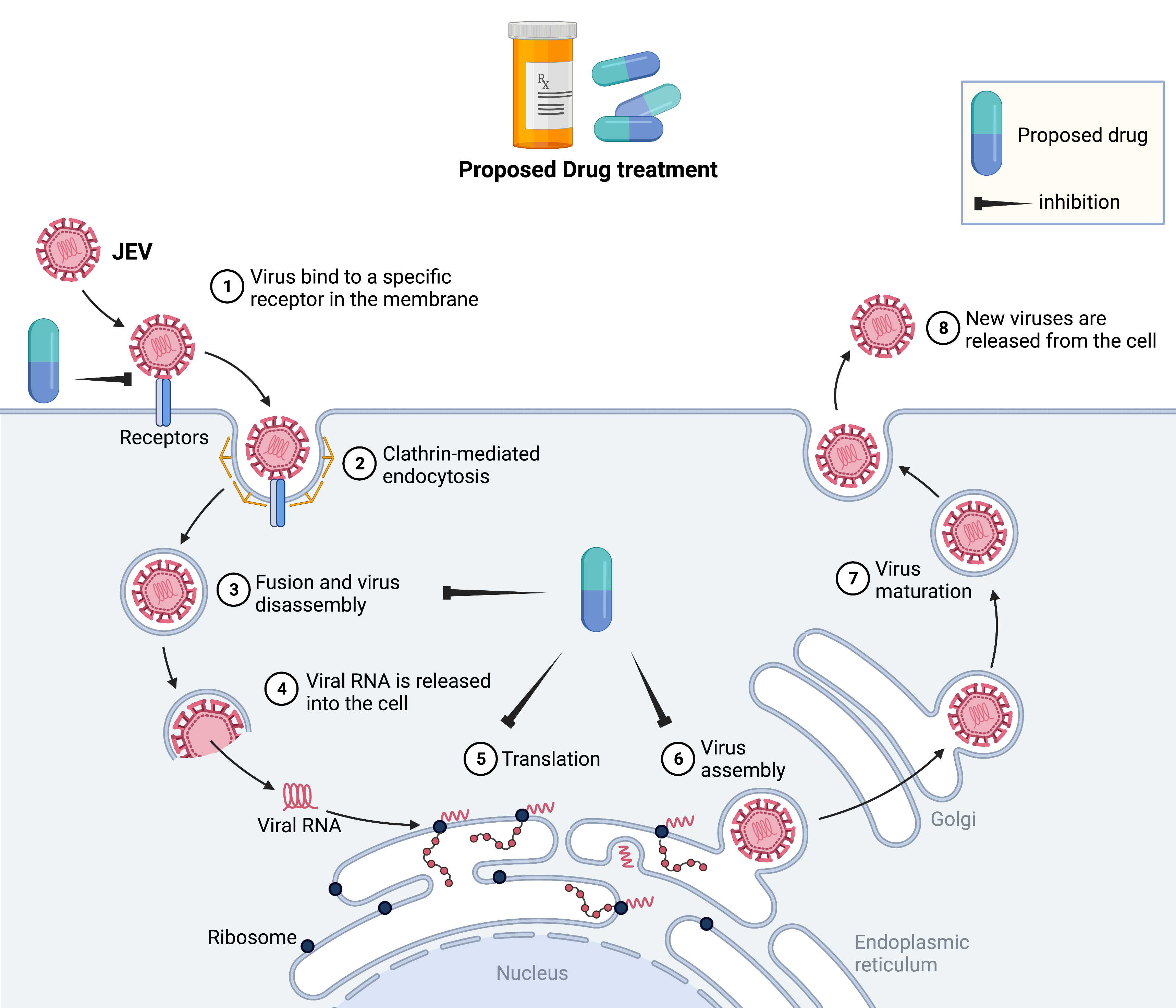
1) Development of novel therapeutics against Dengue and JEV menace:
Under the CSIR-CDRI Virus Mission Program, our laboratory is acting as a key laboratory for the Designing and Development of Antiviral Strategies for Dengue and Japanese Encephalitis (JE) viral infections. India is a hot spot for both viruses since they have >90% antigenic similarity and belong to the same genus Flavivirus.
Among the Flaviviruses, Dengue (break-bone fever) is the more widespread viral disease transmitted through bite of an infected Aedes spp mosquito, affecting half of the world's population with an estimated ~400 million infections annually. Presently, there is no specific treatment for dengue, supporting care is the only option to manage the disease. We at CSIR-CDRI, have adopted a ‘Fast-Follower’ approach to design novel antiviral therapeutics to combat these deadly diseases. Through computer-guided target-based drug discovery technique, we have identified potential hits against putative targets of dengue virus (DENV) from the fully characterised chemical library/natural product resources (figure below). Moreover, these hits are being optimised chemically for better potency and preclinical development.
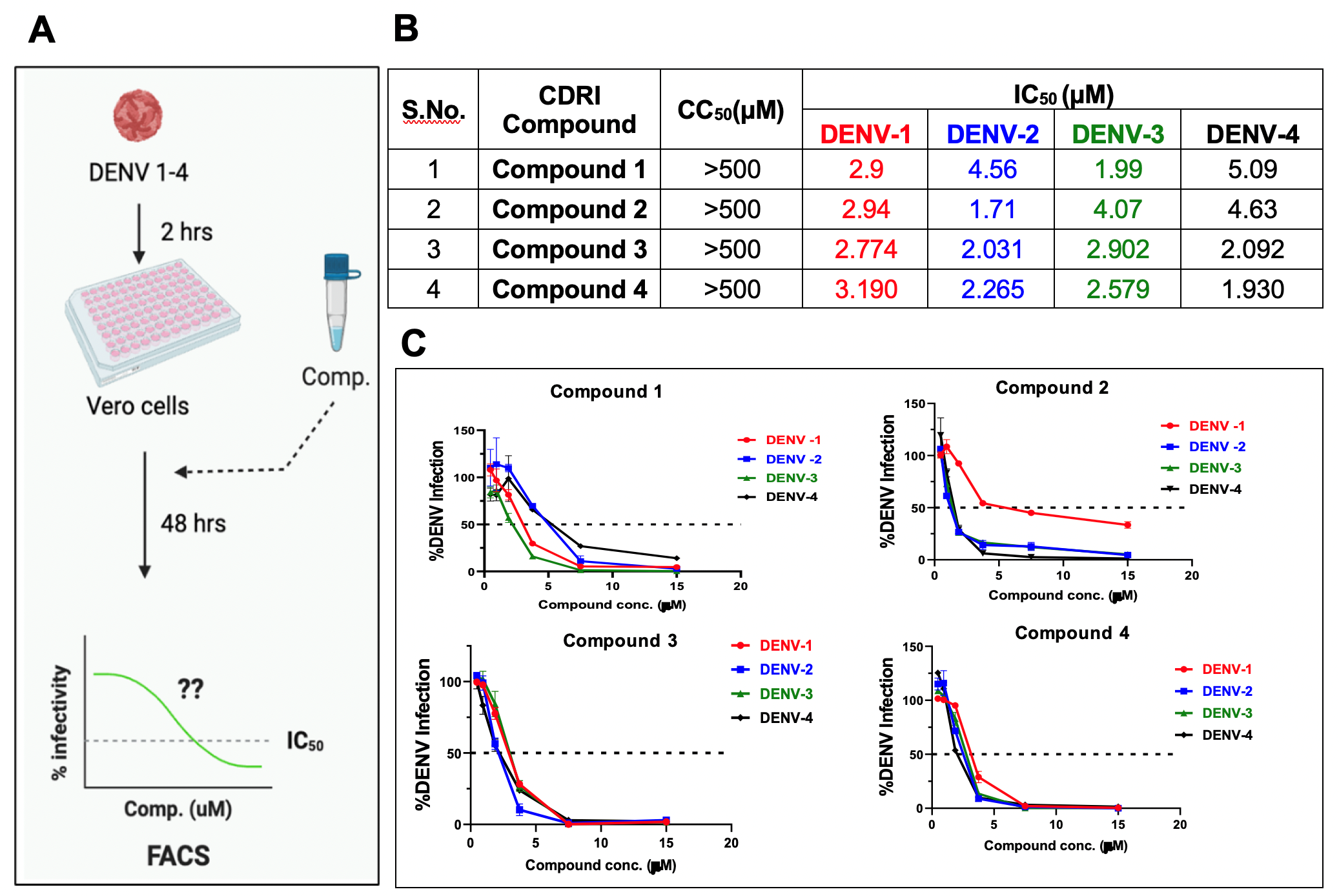
Figure: Identified hits against DENV1-4. (A) Adopted strategy for screening of antivirals through cell-based FRNT, (B) Half maximal inhibitory concentration (IC50) against WHO-referenced all four serotypes of DENV along with their 50% cytotoxic concentration (CC50), (C) Dose-response curve of identified initial hits against DENV1-4.
2) Design and development of neutralising monoclonal antibodies as therapeutics against DENV
Dengue fever is the most prevalent mosquito-borne viral disease globally. Most individuals who are infected with dengue will not experience symptoms. However, for those who do, may lead to develop severe dengue. Severe dengue infections can lead to life-threatening illnesses and the need for immediate medical attention. In the absence of a broad-use vaccine or antiviral drug against dengue, therapeutic antibodies that neutralise DENV may serve as an effective medical countermeasure against severe dengue. However, therapeutic antibodies would need to neutralise all four serotypes of DENV effectively. It must not induce Antibody-Dependent Enhancement (ADE) of DENV infection in monocytes/macrophages through Fc gamma receptor (FcγR)-mediated viral entry, which is the leading risk of severe dengue. Our lab interest is to develop the strategies and technologies that can be adopted to develop antibodies for therapeutic applications. With this potentially promising bulwark against dengue, we used computational tools to identify the putative neutralizing epitopes of DENV1-4, followed by the designing of highly specific antibodies. Furthermore, these tailored antibodies are being produced using the recombinant monoclonal antibody production approach (as shown in the figure below). This innovative strategy is not only bypassing the traditional approaches for developing monoclonal antibodies, but it would also have several inherent advantages, including the fact that these mAbs are mono-specific by design, high-throughput, scalability and consistency in terms of production, and the highest quality in terms of sensitivity and specificity.
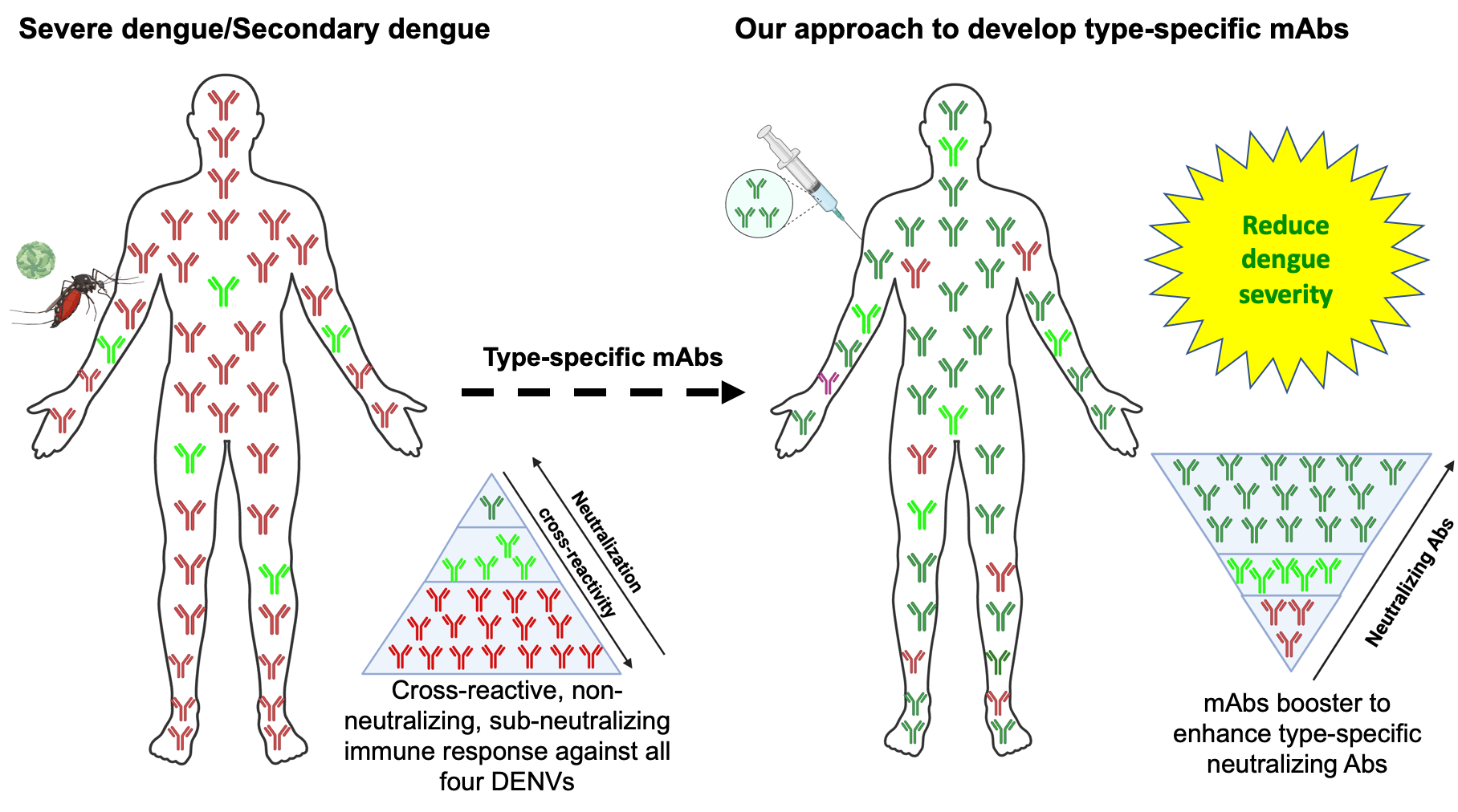
Figure: In a DENV secondary infection, non-neutralising/sub-neutralising/cross-reactive Abs from primary infection binds to the infecting DENV, enhances viral replication through ADE, and worsens the dengue disease as DHF/DSS. To overcome the dengue severe disease, we are developing types-specific neutralising monoclonal Abs (mAbs) against the DENV1-4 serotype to reduce the dengue severity in the clinical setting.
3) Development of safe and cost-effective subunit vaccine candidates against JEV:
With our expertise in developing subunit vaccine candidates against DENV (Ramasamy et at., 2016; Shukla et al., 2020), our group is actively involved in the designing of a Virus-Like Particles (VLPs)-based vaccine candidate(s) against JEV. Our approach to design the ‘tailor-made’ vaccine candidate by customizing the putative-neutralising epitopes of JEV and develop the VLPs which would induce long-lasting humoral as well as cell-mediated immune responses with the inherent safety potential. The available live-attenuated vaccines for JEV have proved efficient in preventing JE with the reports of probable adverse events including from hypersensitivity reactions to the rare cases of terrible effects on CNS (encephalomyelitis and seizures). We at CDRI with the collaboration of Dr. Navin Khanna’s group (ICGEB, New Delhi) are determined to develop a broad spectrum, safe, cost-effective designer subunit-based vaccine candidate against JEV.
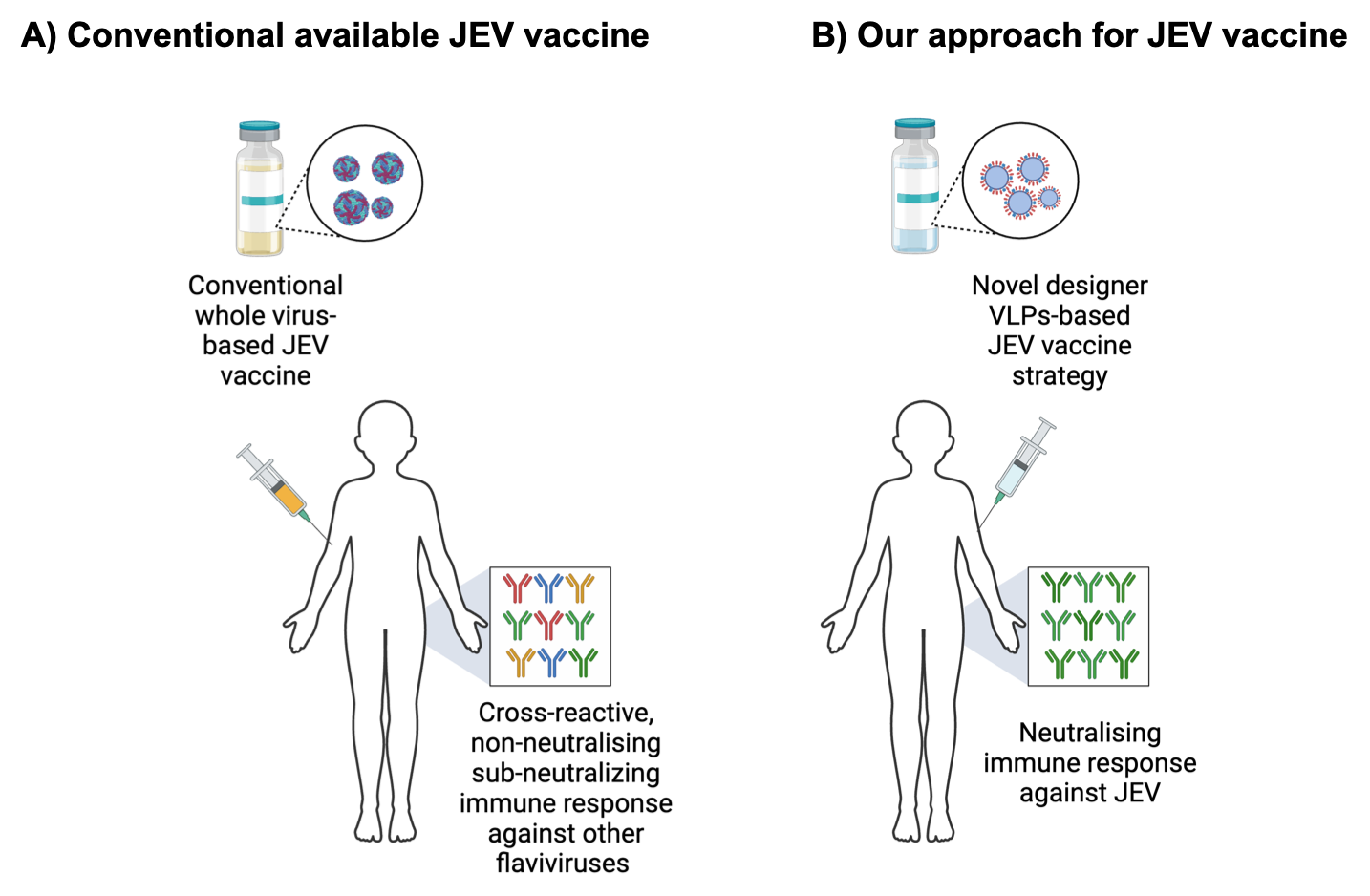
Figure: Comparison of conventional JEV vaccine vs. our approach for developing designer VLPs-based JEV vaccine candidate immune responses.
4) Investigate the biomarkers and immunological responses involved in dengue disease severity and/or in terms of co-morbidity conditions
Dengue virus infection can cause a variety of symptoms, including asymptomatic dengue fever (DF), as well as severe dengue diseases such as dengue haemorrhagic fever (DHF) and dengue shock syndrome (DSS). Early detection of severe dengue in patients with no warning signs who may later develop severe DHF is critical for selecting appropriate intensive supportive care, which is the only available option to manage the disease. Early identification of dengue patients at risk of adverse outcomes is not only important to prevent hospital overcrowding in low- to middle-income countries, but it also important for clinicians to manage the disease in co-morbidity conditions during epidemics. Severe dengue responses include T and B cell activation, proinflammatory cytokine storm, hematologic disorders and complement activation. Cytokines and complement activation, and other unidentified factors which may transiently acts on the endothelial cells which circumvent in blood vascular leakage, is one of the hallmark phenomenon of severe dengue. However, there is no concrete evidence and early predictor biomarkers that could inform the onset of dengue severity and overt the severity process by clinical intervention is lacking.
Most recently, we have successfully established small animal model, AG129 and scored the probable ADE potential in a limited approved dengue vaccine, Dengvaxia (Shukla et al., 2021; https://www.sciencedirect.com/science/article/pii/S2352396420303674) and evidenced that Dengvaxia has enhancement potential not even against DENV but also enhances ZIKA virus infection in vivo. Later this study were linked to the United Nations Sustainable Development Goals, helping to tackle some of the world’s greatest challenges.
By utilising our experience in developing bio models and scoring the ADE potential, we are interested in investigating and identifying the early predictor biomarkers and immunological responses that lead to dengue severe disease. This project aims to evaluate the status of immune cells, levels of proinflammatory cytokines and chemokines, and assess the endothelial activation markers and biochemical markers as the predictor of severe dengue disease, particularly in case of co-morbidity developed by the infection of the related viruses (ZIKV, JEV and SARS CoV-2).