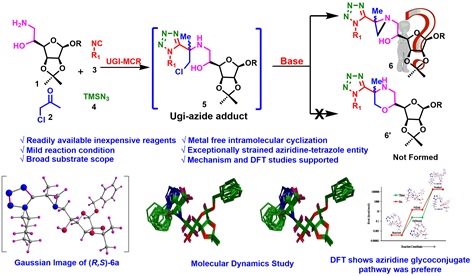
Highlight on unique reactivity associated with glycosyl amino alcohols for synthesis of substituted aziridinyl glycoconjugate
The base-promoted intramolecular cyclization of Ugi-azide adduct has been demonstrated for the synthesis of highly
substituted aziridinyl glycoconjugates in one pot. The reactions are scalable and efficient and have an operationally simple
broad substrate scope. To gain insight into the mechanism of aziridine formation, DFT and control experiments show that the
cyclization of the aziridine glycoconjugate pathway was preferred, as it proceeds with a low activation energy barrier
(0.57 kcal mol–1), which supports our experimental observation. (Organic Letters, 2019, 21, 2859-2862).
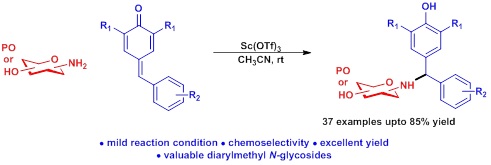
Scandium triflate-catalyzed 1,6-conjugate addition of unprotected carbohydrate amines to para-quinone methides: expedient access
to α,α′-diarylmethylated N-glycosides
Reported herein is a practical route to access the synthetically challenge and chemoselective α,α′-diarylmethylated N-glycosides
derivatives via Sc(OTf)3-catalyzed 1,6-conjugate addition of amino sugars with para-quinone methides (p-QMs). The reactions proceed
smoothly with variety of protected as well as unprotected amino sugar derivatives, avoiding the utilization of any base. This
protocol provides us to access good protecting group tolerance and broad substrate scope in moderate to good yield.
(Org. Biomol. Chem. 2020, 18, 1343-1348)
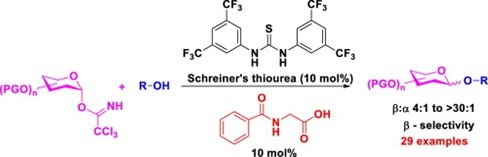
Cooperative catalyzed stereoselective O-glycosidation: Activation of O-glycosyl trichloroacetimidate donors
A new practical utility for β-stereoselective glycosylation via activation of O-glycosyl trichloroacetimidate donors using
N-benzoylglycine/thiourea cooperative catalysis has been demonstrated. This method represents the first instance where amino
acid derived N-benzoylglycine is used as a catalyst for O–glycosylation under mild reaction conditions at ambient temperature.
NMR spectroscopy studies suggest that thiourea cocatalyst exhibit a cooperative behaviour that has a strong effect on the
reaction rate, yield, and the β-selectivity. (Catal. Commun, 2019, 125, 123-129)
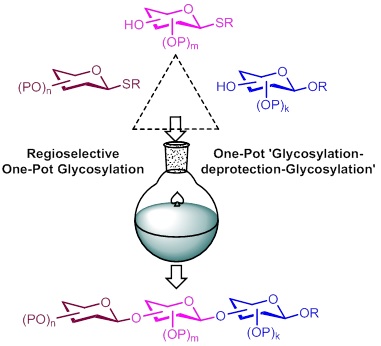
One-Pot ‘Glycosylation-deprotection−Glycosylation’ Strategies for synthesis of Complex biologically relevant oligosaccharides and glycoconjugates
Our notable contributions towards synthesis of biologically relevant oligosaccharides usin one−pot glycosylation, and one−pot deprotection−glycosylation
protocols.(Tetrahedron Letters 2019, 60, 230-234, Tetrahedron Letters 2019, 60, 151153, Tetrahedron Letters 2019, 60, 860-863, RSc Advances 2016,
6, 7736-7745. Synlett 2016, 27, 2581-2586. Synthesis 2015, 47, 836-844. Tetrahedron Letters 2015, 56, 900-902. Beilstein J. Org. Chem. 2014, 10,
2724–2728. Beilstein J. Org. Chem. 2014, 10, 293-299).
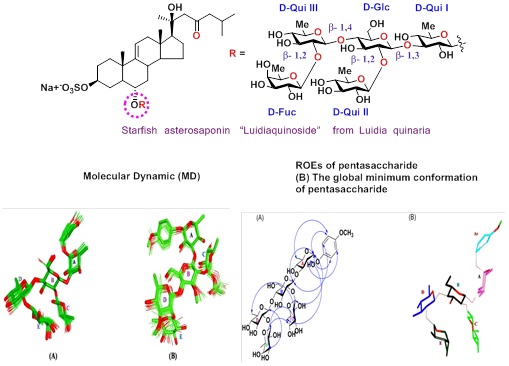
Synthesis of the Pentasaccharide Moiety of Starfish Asterosaponin Luidiaquinoside and its Conformational Analysis.
The synthesis of the pentasaccharide moiety of starfish asterosaponin luidiaquinoside as their p-methoxyphenyl (PMP) glycosides
was achieved followed by sequential glycosylation strategy using suitably functionalized thioglycoside donors. To gain an
understanding about the structural properties of these pentasaccharides in a biologically relevant environment, NMR studies
were carried out in aqueous solution and the restrained molecular dynamics (MD) were carried using the experimental restraints
derived from the NMR studies. (RSc Advances, 2016, 6, 7736-7745).