Polyamine biosynthesis pathways
Polyamines including putrescine, spermidine, spermine are essential cellular components involved in a multitude of physiological processes. Kinetoplastids including Leishmania utilize trypanothione (bis glutathione spermidine) in neutralizing ROS during host-pathogen interactions. Enzymes of this pathway are of therapeutic interest and our group has initiated characterization from this pathway. Enzymes that perform successive steps in a pathway associate as a complex, known as a metabolon. One such metabolon identified by the group is the Spermidine Synthetase:S-adenosyl methionine decarboxylase (Spdsyn:adometDC) complex. Computational analysis indicate that this complex is distinct from the human homologous metabolon and structural characterization is in progress
Purine salvage pathway
Purines are involved in various cellular functions including cell signaling, growth, energy production, synthesis of vitamins/cofactors. L. donovani, lack the de novo purine synthesis pathway and hence are dependent on the purine salvage pathway. Transported nucleobases are incorporated into the nucleotide pools via a complex purine salvage pathway. The group has characterized and identified inhibitors L. amazonensis Nucleoside Diphosphate Kinase (NDK), in collaboration with Prof. K.P. Chang’s group and currently the focus is on other enzymes of the salvage pathway.
Understanding principles of helical assembly
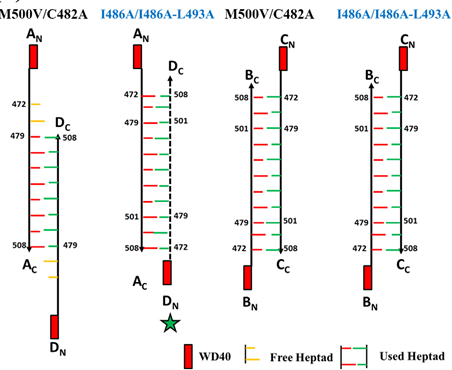
Protein-protein interactions, vital for cellular functions are mediated by a variety of domains including the Coiled-coil (CC) domain. In the CC, the interacting helices associate in a knobs into holes manner, mutually burying their hydrophobic residues, resulting in a superhelix with specific handedness. CCs are inherently elastic, and this could be the prime reason for this motif being observed ubiquitously. The interacting helices have been shown to axially move with respect to each other, the movement potentially in the range of a few Ås to a nm. The interacting helices can also ‘rotate’ or undergo scissoring motions. The elucidation of the mechanisms that guide these motions are of interest to the group, using the coiled-coil domain of the actin bundling protein coronin. Structure elucidation of the L.donovani coronin CC showed a novel topology and oligomer association, and possessed a striking asymmetry, with one of the helices of the four helical bundle ~axially out of register with the other helices by ~a nm. Analysis identified the symmetric bundle is sterically hindered, that was confirmed with the structure determination of the mutant CC.