Post-IMCR Modifications and Total Synthesis
Our lab is working on the development of post-IMCR modification strategies for the construction of uniquely functionalized scaffolds of biological interest. Notable publications from our research group in this area are highlighted below:

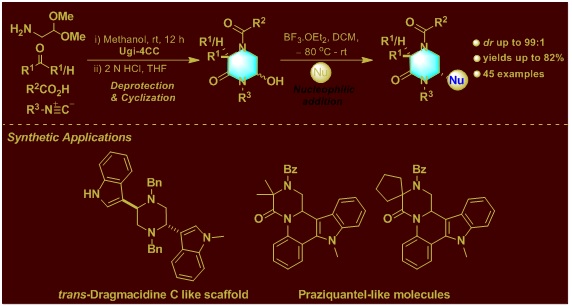
1. Construction of Highly Functionalized Piperazinones via post-Ugi Cyclization and Diastereoselective Nucleophilic Addition.
J. Org. Chem. 2020, 85, 6910–6923.
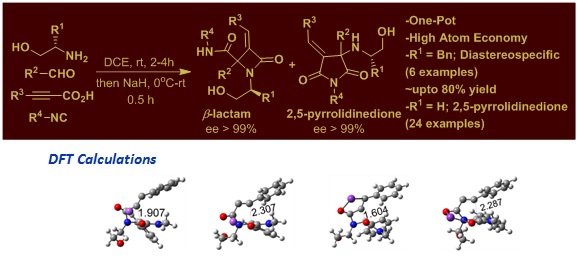
2. Asymmetric Synthesis of Functionalized 2,5-Pyrrolidinediones and β-Lactams through Diastereospecific Cycloisomerization/ Rearrangements of Chiral Ethanolamine Derived Ugi Adducts.
Eur. J. Org. Chem. 2017, 2245–2257.
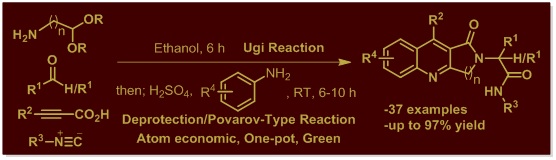
3. An Efficient One-Pot Synthesis of Densely Functionalized Fused-Quinolines via Sequential Ugi4CC and Acid-Mediated Povarov-Type Reaction.
ACS Comb. Sci. 2017, 19, 600–608.
4. Alkaloid-Mimicking Tricyclic Skeletons by Diastereo- and Regioselective Ugi/ipso-Cyclization/Aza-Michael Cascade Reaction in One-Pot.
Org. Lett. 2016, 18, 1040–1043.
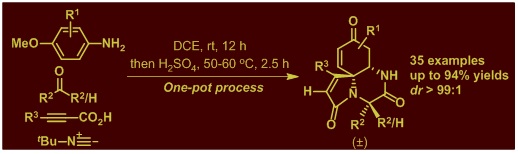
5. Efficient Construction of Azaspiro[4.5]trienone Libraries via Tandem Ugi 4CC/Electrophilic ipso-Iodocyclization in One-Pot.
ACS Comb. Sci. 2015, 17, 474–481.
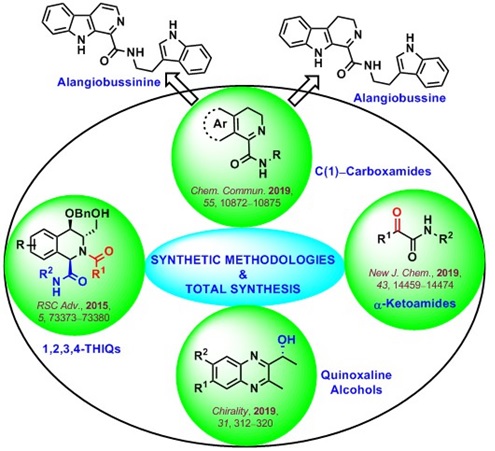
Synthetic Methodologies and Total Synthesis
Our lab has developed several synthetic methodologies in the recent years and their possible utilization for the total synthesis of bioactive natural products has also been explored. Key contributions from our research group in this area are highlighted below:
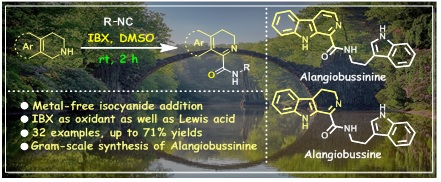
6. IBX-mediated oxidative addition of isocyanides to cyclic secondary amines: Total syntheses of alangiobussine and alangiobussinine.
Chem. Commun. 2019, 55, 10872–10875.
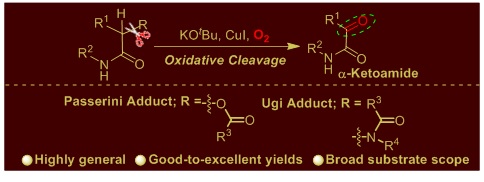
7. Copper-catalyzed oxidative cleavage of Passerini and Ugi adducts in basic medium yielding α-ketoamides.
New J. Chem., 2019, 43, 14459–14474.
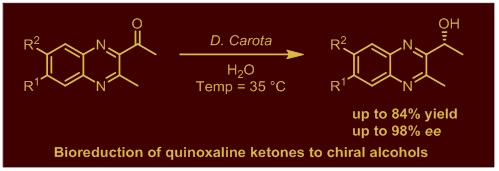
8. Green synthesis of enantiopure quinoxaline alcohols using Daucus carota.
Chirality, 2019, 31, 312–320.
9. Stereoselective synthesis of functionalized 1,2,3,4-tetrahydroisoquinolines (THIQs) via highly diastereoselective Ugi three-component reactions (U3CRs) with chiral 3,4-dihydroisoquinolines (DHIQs).
RSC Adv., 2015, 5, 73373–73380.

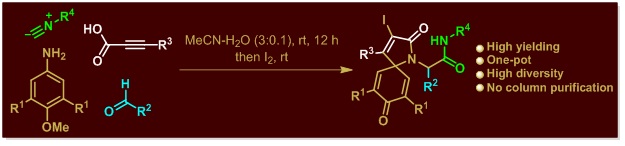
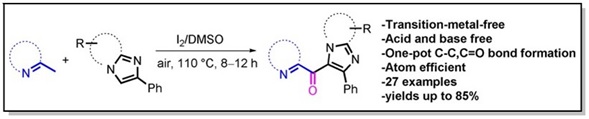
10. Transition‐Metal‐Free Oxidative Cross‐Coupling of Methylhetarenes with Imidazoheterocycles towards Efficient C(sp2)−H Carbonylation.
Asian J. Org. Chem., 2017, 6, 890–897.
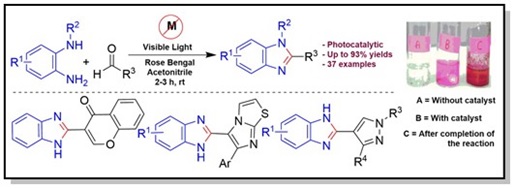
11. An Efficient Synthesis of 2-Substituted Benzimidazoles via Photocatalytic Condensation of o-Phenylenediamines and Aldehydes.
ACS Comb. Sci. 2016, 18, 644–650.
Medicinal Chemistry
Our lab is currently working on different medicinal chemistry projects to identify novel therapeutic leads for obesity, cancer, hyperlipidemia
and tuberculosis. We also use modern computational methods such as QSAR, docking, molecular dynamics simulation studies to design virtual
hits that are further taken for synthesis and biological evaluations. Key contributions from our research group in this area are highlighted
below:
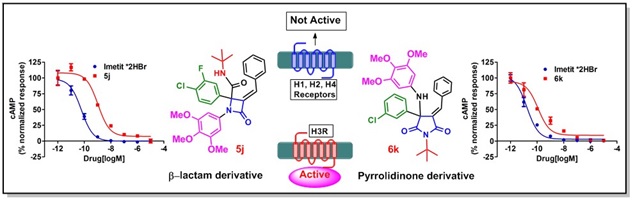
12. Identification of novel β-lactams and pyrrolidinone derivatives as selective Histamine-3 receptor (H3R) modulators as possible anti-obesity agents.
Eur. J. Med. Chem., 2018, 152, 148–159.
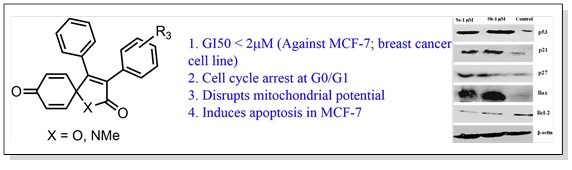
13. Design, synthesis and anticancer properties of novel oxa/azaspiro[4,5]trienones as potent apoptosis inducers through mitochondrial disruption.
Eur. J. Med. Chem., 2015, 101, 348–357.
14. Investigation of podophyllotoxin esters as potential anticancer agents: Synthesis, biological studies and tubulin inhibition properties.
Eur. J. Med. Chem., 2015, 89, 128–137.
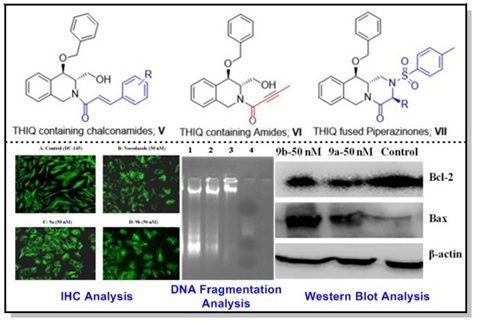
15. Design, synthesis and biological evaluations of chirally pure 1,2,3,4-tetrahydroisoquinoline analogs as anti-cancer agents.
Eur. J. Med. Chem., 2015, 92, 608–618.
16. Development of constrained tamoxifen mimics and their antiproliferative properties against breast cancer cells.
Bioorg. Med. Chem. Lett., 2015, 25, 680–684.
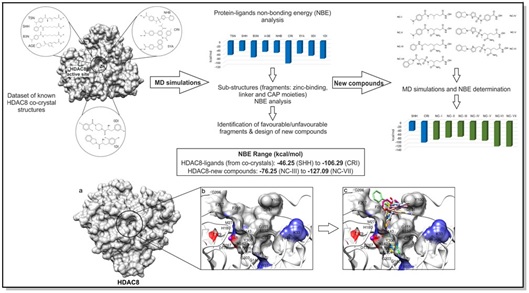
17. Investigation of HDAC8-ligands’ intermolecular forces through molecular dynamics simulations: profiling of non-bonding energies to design potential compounds as new anti-cancer agents.
J Biomol. Struct. Dyn., doi.org/10.1080/07391102.2020.1780940.