Our lab is proficient in various approaches for development and characterization (in-vitro and in-vivo) of novel drug delivery systems including nanoparticles, nanoemulsion, miceller systems, nanocrystals, and liposomes. Apart from regular pharmaceutical handling (manufacturing, characterization, evaluation, dissolution, stability, morphological, quality by design, etc.), we explore several cell culture based assays, fluorescence microscopy, flow cytometry. We have been engaged in preclinical drug development program, and hence have to handle various animal models for pharmacokinetic, pharmacodynamic and bio-distribution studies.
At CSIR-CDRI, we have had the good fortune of interacting with experts and scholars from diverse fields of science and consequently we realize that ‘how’ and ‘why’ are pillars of equal footing to the often celebratory pharmacokinetic and pharmacodynamic implications of a delivery system. Consequently we accommodate multidisciplinary approaches to develop and validate a drug delivery system for pathophysiological condition
Sampled here is a brief foray of the type of work carried in our lab. We have instrumentation to auger oral, parenteral, sub cutaneous, transdermal, depot, in situ gelling formulations for a variety of drug candidates. We are not hooked to any particular strategy or delivery aspect and instead focus on the possibility, plausibility and applicability of a delivery system and the drug which it carries.
Delivering Anticancer Drugs
Docetaxel
Docetaxel is a favoured option for breast cancer treatment; however its marketed formulation (Taxotere) generates therapeutic response at the cost of undue toxicity. In order to circumvent such limitations, nanocrystals were prepared using high pressure homogenization technique using pluronic F-127 as a stabilizer. Nanocrystals presented higher efficacy against MCF-7 breast cancer cells with exposition and enhanced the drug induced G2-M arrest and apoptosis. The claims of improvement were substantiated by investigating the modulation in apoptotic mechanism induced by the subtle physical state variation of drug in nanocrystals. Results revealed that nanocrystal induced apoptosis was linked to altered mitochondrial membrane potential. Safety of nanocrystals was ascertained via haemolytic testing and in-vivo toxicity studies in mice. Developed formulation exhibited acceptable haemolytic potential which suggested its suitability towards parenteral administration. Moreover, in-vivo acute toxicity studies demonstrated that the developed nanocrystals were safer than marketed formulation. These results elicit that developed nanocrystals would be a viable alternative to commercial formulation for treatment of breast cancer
Paclitaxel
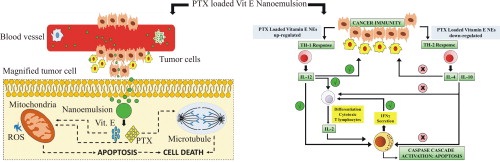
Paclitaxel is used as first line treatment for metastatic breast cancer but the relief comes at a heavy cost in terms of accompanying adverse effects. The pharmaceutical credentials of paclitaxel are further dampened by the intrinsically low aqueous solubility. In order to sideline such tendencies, paclitaxel was incorporated in a vitamin E nanoemulsion using high pressure homogenization. The emulsion had a high drug entrapment efficiency and sustained drug release profile. The nanoemulsion exhibited higher cytotoxicity MCF-7 cells. Cell cycle arrest study depicted that nanoemulsion showed high arrest in G2-M phase. Moreover blank nanoemulsion induced additional apoptosis in breast cancer cells through G1-S arrest by disrupting mitochondrial membrane potential. Cytokine estimation study in macrophages showed that both nanoemulsion enhanced secretion of IL- 12 and downregulated secretion of IL-4 and IL-10. Results suggested that inclusion of vitamin E in nanoemulsion opened multiple complementary molecular effects which not only magnified the Principal antiproliferative activity of paclitaxel but also independently showcased potential in restoring the proactive nature of the breast cancer slackened chronic immune response. In-vivo anticancer activity showed significantly improved efficacy of. The list of plausible advantages of nanoemulsification was further substantiated by acceptable haemolytic potential, reduced in-vivo toxicity and conveniently modified pharmacokinetic profile in which the AUC and MRT were extended considerably.
Bendamustine delivery entailed complexation of oppositely charged carbohydrates sodium aliginate and chitosan inside nanoreactor assemblies housed inside reverse micelles Dynamic light scattering, electrophoretic mobility measurement and UV spectroscopic experiments were used to investigate the dynamics of nanoscopic intra -miceller synthetic units and to confirm that drug resided inside the core of reverse micelles and underwent concurrent complexation with charged polymers. The developed complexation nanoparticles have minimal haemolytic potential and provide sustained in-vitro drug release for up to 48 h which results in almost 40% reduction in IC50 enhancement in G2M cell cycle arrest and increase in early and late apoptotic potential of drug. Quantitative and mechanism based cell uptake studies further reveal that monocytes burdening the blood in leukaemia have voracious capability to internalize the developed nanoparticles via simultaneous scavenger receptor based endocytosis and phagocytosis mechanism. A fluorescent probe rhodamine 123 conjugate with dug was synthesized via carbodimide coupling and was used to determine the actual fate of drug after its loading into carbohydrate nanoparticles. Confocal microscopy unequivocally demonstrated that developed nanoparticles offered enhanced protection ferrying drug directly to its site of action i.e. nuclei. Further, comparative in-vitro kinetic studies in THP-1 cells revealed obtainment of significantly greater intracellular drug levels.
The list of anti-cancer drugs prescribed here is not exhaustive as we are pursuing delivery candidates for several other drugs like doxorubicin, gemcitabine, curcumin, andrographolide, methotrexate, metformin, etc for targeting variety of cancers.
An open mike for pitching ideas against parasitic infections
Amphotericin
Our lab has worked exhaustively on Amphotericin B delivery. We have utilized multiple entry points. Strategies include polymeric micro particles, solid lipid nanoparticles, hybrid nanoparticles, nanocapsules, polymeric micelles. These systems exploit advertent passive or active targeting to resident macrophages which house the infectious parasites sometimes in conjunction with immunomodulatory effects to achieve effective killing. Since amphotericin B is extremely toxic special emphasis has been laid on evaluating circulatory and morphology based pathological markers. Plasma kinetics and organ distribution studies further supplement the probable effects of the developed delivery system, whose efficacy is finally evaluated in infected hamsters.
Lymphatic targeting of filarial parasites
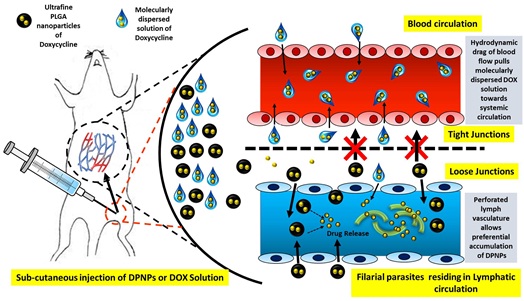
Systemic chemotherapeutic targeting of filarial parasites is unfocussed due to their deep seated location in lymphatic vessels. This warrants prolonged dosing regimen in high doses for an anthelminthic like Doxycycline hydrochloride. In order to provide an alternative, we constructed ultrafine PLGA nanoparticles of doxycycline so as to exploit the peculiarity of lymphatic vasculature underneath sub cutaneous layer of skin, which preferentially allows entry of only 10-100nm sized particles. Nanoparticles have been constructed using a novel solvent diffusion method aided by probe-sonication. TEM substantiated the spherical shape of the nanoparticles along with their actual non hydrated size being well below 100 nm. Pharmacokinetic studies in Wistar rats further revealed that nanoparticles caused a 16 fold prolongation in attainment of plasma Tmax and a 2 fold extension of elimination half-life of doxycycline. Contrastingly the trend was reversed in regional lymph nodes where. This size based preferential lymphatic targeting resulted in significantly greater in-vivo antifilarial activity of nanoparticles as gauged by several parameters in B. malayi infected M. coucha
Dual or triple mechanism based multi particulate gastroretentive systems have been developed and exploited to tackle half-life issues of susceptible drugs (norfloxacin, capecitabine). The emphasis here lies in core pharmaceutics, i.e. developing well rounded reproducible microparticles which readily exhibit the desired characteristics of floatation and/or mucoadhesion and sustained release. The approach is varied in exploiting GRAS excipients, but the optimization process tethers in the domain of scientific experimental design with limited literary and hit and trial inputs. Surface morphology and solid state characterization have been an important aspect in predicting the drug release patterns and over all behaviour of system in bio relevant conditions.
RESEARCH GRANT RECEIVED:
1. Grant-in-aid project entitled “Investigation of effect of polysaccharide in modifying leishmanicidal potential of nanoparticulate system bearing chemotherapeutics agent”
Funded by: Department of Biotechnology [Oct 2011 to Oct 2014]
2. Grant-in-aid project entitled “Target oriented delivery of chemotherapeutic agent in leishmaniasis via macrophage scavenger receptors”
Funded by: Department of Science and Technology [May 2014 to May 2017]
3. Grant-in-aid project entitled “Design, development and performance evaluation of hybrid systems comprising novel cationic lipids intended to deliver therapeutic siRNA to solid tumors”
Funded by: Department of Biotechnology [Feb 2016 to Feb 2019]