Understanding the role of RNA Polymerase II CTD for the mRNA transcription
Eukaryotic transcription by RNA polymerase II is accomplished by a fine-tuned interplay along with chromatin remodeling complexes,
RNA transcription factors and RNA processing factors. RNA polymerase II uniquely possesses an extended carboxy terminal domain (CTD) on its
largest subunit, Rpb1, comprising a repetitive Tyr1Ser2Pro3Thr4Ser5Pro6Ser7 motif with potential epigenetic modification sites. The changes in
CTD phosphorylation observed in response to cell cycle regulation events and to the extracellular stimuli raise the intriguing possibility that
CTD serves as a signal for the binding of various transcription regulators essential for mRNA biogenesis and export. A growing body of evidence
suggests that apart from the proteins involved in transcription, many proteins associated with CTD participate in DNA metabolism,
protein synthesis and turnover, chromatin remodeling etc. The above observations suggest that the functional repertoire of CTD is great and
it is important to identify the physical connection of eukaryotic proteome to CTD and its function.
It has long been recognized that transcription is silenced during mitosis due to the presence of highly condensed chromatin and dissociation and deactivation
of a few key components of the transcription complex. However, a few recent studies support active transcription albeit with a distinct mechanism. Conversely,
active transcription was also speculated by findings of de-condensed chromatin fibrils, RNAPII associated transcription factors, phosphorylated CTD, mitotic
CTD phosphatase, PTEFb dependent mitotic transcriptional activation etc. We have identified a mitotic CTD kinase (Cdc15) whose inactivation affects CTD
phosphorylation and ongoing transcription. To what extent and the mechanism by which RNAPII complex can direct active transcription in mitosis remain
unknown and is a current focus of our research. A timely exit of the cells from mitosis is essential and failure of cell cycle check points and prolonged
mitotic arrest induces genetic instability and mitotic cell death. Hence it is utmost important to understand the mechanism of gene regulation during
mitosis.
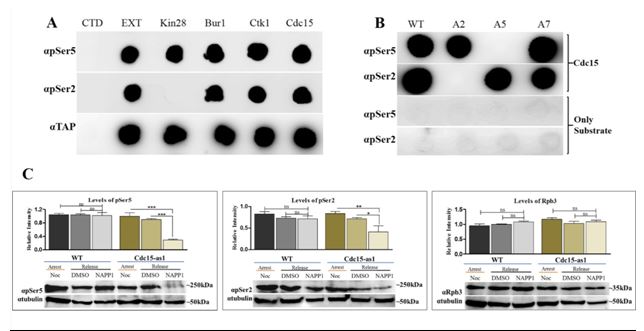
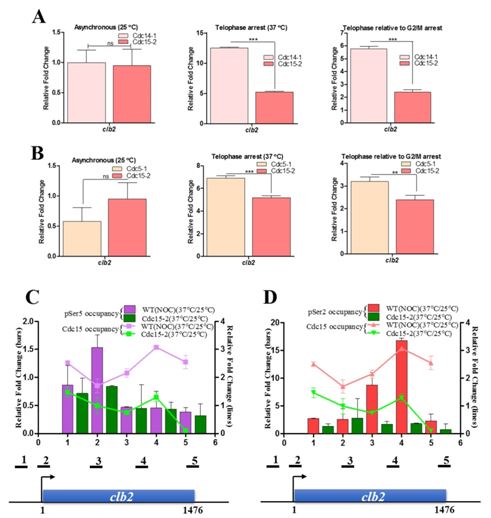
(A) We have identified Cdc15 as a potential kinase phosphorylating Ser2 as well as Ser5 of CTD for transcription during mitosis in the budding yeast. The phosphorylation of CTD by Cdc15 is independent of any prior Ser phosphorylation/s. The inactivation of Cdc15 causes reduction of global CTD phosphorylation during mitosis and affects the expression of genes whose transcript level peaks during mitosis. Cdc15 also influences the complete transcription of clb2 gene and phosphorylates Ser5 at the promoter and Ser2 towards 3end of the gene. The observation that Cdc15 could phosphorylate Ser5 as well as Ser2 during transcription in mitosis, is in contrast to the phosphorylation marks put by the kinases in interphase (G1, S and G2), where Cdck7/Kin28 phosphorylates Ser5 at promoter and Bur1/Ctk1 phosphorylates Ser2 at 3' end of the genes.
a
b
(B)
Our work involves the development of model to study the in vivo interaction of proteins due to the epigenetic modification of CTD and the identification of CTD
interacting transcription factors/regulators and their subsequent characterization for essential cellular functions. One such example is
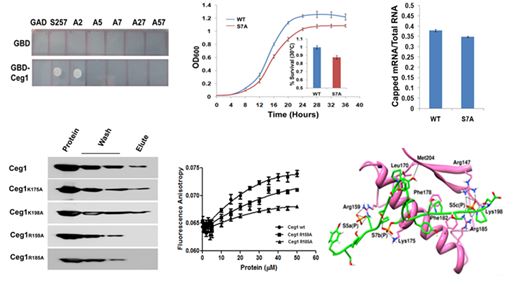
the mRNA capping enzyme of Saccharomyces cerevisiae, which appears to have dual specificity and interacts with the phosphorylated Ser5 and
Ser7 of CTD. The Ser7 of CTD is essential for the unconditional growth and the efficient mRNA capping. The Arg159 and Arg185 of RNA
guanylyltransferase (Ceg1) are the key residues that interact with the Ser5P, while the Lys175 with Ser7P of CTD. These interactions appear
to be in a specific pattern of Ser5PSer7PSer5P in a tri-heptad CTD (YSPTSPPSYSPTSPSPYSPTSPPS) and provide molecular insights into the Ceg1-CTD
interaction for mRNA transcription.
Structure-function relationship of phage hyaluronatelyase
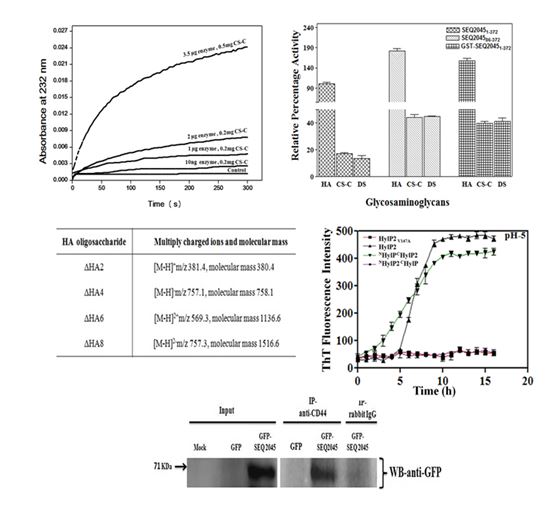
The limited specificity of phage hyaluronatelyase (HL) of Streptococcus equi (S. equi)and its lack of extracellular secretion suggest its role is restricted for bacterial lysogenization. Conversely, the antibody response to phage HLs in the case of S. equiand S. pyogenesduring in vivo infection has also been observed and hint that the role of phage HL may also in conferring virulence to the host bacterium. Here we systematically looked into the structure-function relationship of three phage HLs. While HA is the preferred substrate, these HLs have weak activity towards CS-C and DS and can completely degrade all of them. Even though the catalytic triple-stranded β-helix (TSβH) domain of phage HL is functionally independent, its catalytic efficiency and specificity is influenced by the N-terminal domain. The phage HL also interacts with human transmembrane glycoprotein CD44. The above results suggest that the streptococci can use phage HLs to degrade GAGs of ECM for spreading virulence factors and toxins, while utilizing the disaccharides as a nutrient source. We also identified a ‘hot spot’ region, influencing the fibril formation in bacteriophage encoded hyaluronatelyases (HylP and HylP2). Here, the N-terminal globular domain appears to initiate the fibrillation whereas, the C-terminus containing ‘hot spot’ region accelerate the fibrillation.
Our lab focuses on the complete characterization of the mechanism of action of phage hyaluronatelyases and their digestion products. The subsequent point mutations in the catalytic pocket or at the substrate binding regions would help us to get the desired enzyme and saccharides mers for commercial purposes.