Design and Synthesis of antiinfective agents
• Search for new antimicrobials, antimalarials and antileishmanials
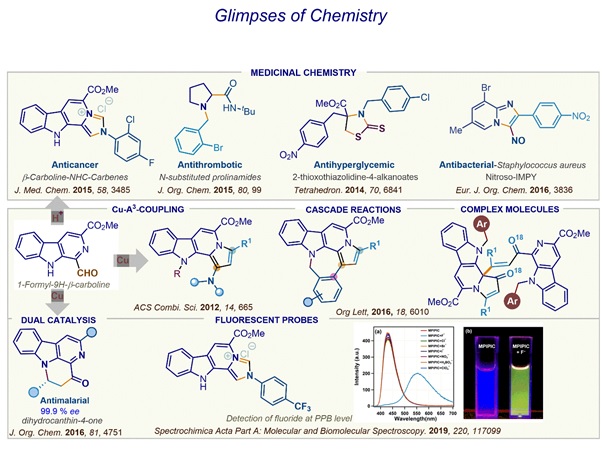
Infective diseases qualify to be a global problem as the current chemotherapy falls short due to several issues majorly being the resistance of pathogens. Consequently, new chemicals to treat such diseases are urgently required. In this context, over the years we have been working towards design and assessment of chemical libraries of novel heterocyclic and alicyclic compounds to identify leads as a prelude to discovery new chemical compound for drug development. For the design of new molecules our group relies on both structure-based approach and serendipity. Our design encompasses novel structures, installing of knowledge-based pharmacophores or preparing hybrid molecules.
Repositioning of bioactives for new leads
Repositioning is a unique paradigm in the area of drug research as it
allows identification and development of new indications for existing drugs or biologics. This strategy has
become an increasingly attractive option especially in the area of orphan and antiparasitic diseases. Towards
this approach, we follow bioinformatics-based approach to identify hits from the library of compounds available
to us via in vitro assays. Thereafter, exploration of the chemical space around the hit molecule is performed
to arrive at a lead candidate.
Antileishmanial
Leishmaniasis is a neglected disease caused by protozoan parasite of genus Leishmania. The major clinical forms of this disease are
cutaneous leishmaniasis (CL) caused by L. major, mucocutaneous leishmaniasis (MCL) caused by L. braziliensis, and fatal visceral
leishmaniasis (VL) caused by L. donovani. Most recent World Health Organization (WHO) report indicates that 310 million people are
at risk of contracting leishmaniasis, while 0.7–1 million new infections and 26,000 to 65,000 deaths take place annually. In Indian
subcontinent Visceral Leishmaniasis is widely prevalent specially in the areas of Bihar , Jharkhand and eastern Uttar Pradesh. The
chemotherapy of VL includes antimony-based Sodium Stibogluconate, antifungal drug Amphotericin and its liposomal preparation
Amphotericin B, aminoglycoside paromomycin and anticancer drug Miltefosine. However, the chemotherapy is typically characterised with
parenteral administration, toxicity and problem of resistance with parasite. Miltefosine an oral option is highly teratogenic and
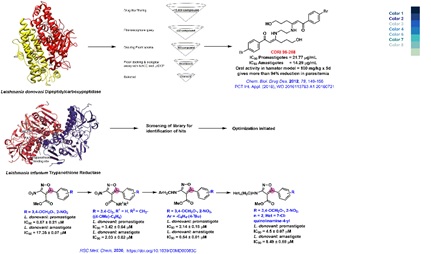
requires 28 days of administration. In this context, the major focus of our group has been to identify an orally active antileishmanial
using both the structure-based design and the random approach. For structure based design we have been targeting the Leishmanial
dipeptidycarboxypeptidase and Trypanothione reductase. We have identified compound 98-288 as an orally active agent with excellent
potency. Subsequently our group has identified another compound 96-261 as an orally active antileishmanial agent. Currently we are
progressing towards IND filing studies with the compound.
Antimalarials
Malaria is an infectious disease caused by a parasite of the genus Plasmodium, and the emergence of parasites resistant to all current antimalarial drugs highlights the urgency of having new classes of molecules. In this context, our group has been working mostly through open science approach. In an open project mode, we participated in the lead optimization studies of an aryl pyrrole derivative initiated by Prof. Matthew Todd (The University of Sydney, Sydney). The result of the study are included in a joint publication ACS Cent. Sci. 2016, 2, 687–701 https://doi.org/10.1021/acscentsci.6b00086.
We are presently working jointly with MMV in an open source drug discovery programme aiming to deliver preclinical candidates for the
treatment and prevention of malaria wherein we are optimizing two leads to find a candidate with better DMPK
profile. For details refer to https://www.mmv.org/mmv-open/malaria-libre/about-malaria-libre.
Bioorg. Med. Chem. 2003, 11(10), 2293-2299
J. Enzyme Inhibit Med. Chem. 2007, 22, 327-342
Bioorg. Med. Chem. 2009, 17, 203-221.
Bioorg. Med. Chem. 2009, 17, 222-234.
Eur. J. Med. Chem. 2009, 44, 4404-4412.
Bioorg. Med. Chem. Lett. 2014, 24, 1719-1723
Parasitology 2016, 143(11), 1421-32.
Tetrahedron 2017, 73, 5680-5689.
Antimicrobials
Finding new antibiotics remains a challenge. Several urea derivatives are known to display excellent antimicrobial and antiproliferative efficacy. Indeed several urea derivatives are being used in clinics to these ailments. As a routine or group have been exploring newer chemotypes for antibacterial efficacy. In this context, we have disclosed potent efficacy in urea derivatives derived from allyl amines. In extension to the study we are now exploring the efficacy of new allyl ureas in resistant bacterial strains.
Bioorg. Med. Chem. Lett. 2006, 16, 3824-3828
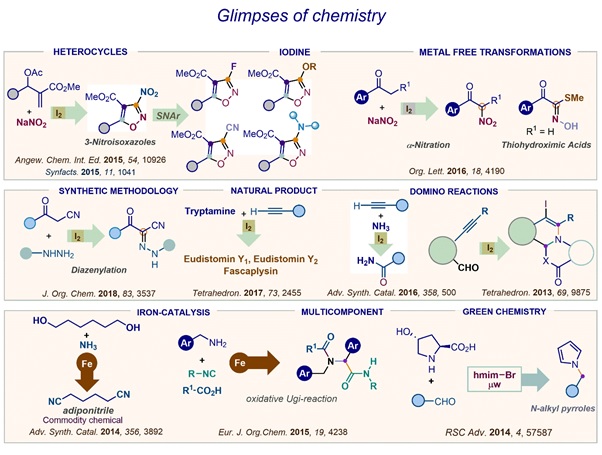
Development of chemistry associated with the Morita-Baylis-Hillman reaction
The Morita-Baylis-Hillman (MBH) reaction is a unique complexity generating organocatalytic reaction between an activated alkene and
carbonyl electrophiles under the influence of a nucleophilic species providing a simple and convenient method for the synthesis of
densely functionalized products. First reported in 1968 by Morita [Bull. Chem. Soc. Jpn., 1968, 41, 2815] as carbinol addition reaction
followed by patents from Baylis and Hillman in 1972 [German Patent 2155113, 1972; Chem. Abstr., 1972, 77, 34174q], this reaction has
grown exponentially especially during the last two decades and has now become a standard method in the toolbox of the synthetic organic
chemists. Some of the hallmarks of this C-C bond forming reaction, which is generally performed using cheap and commercially available
starting reagents, include atom-economy, ease of execution and chemospecific groups in the product for further synthetic
diversifications. At least four different chemical groups in the MBH adduct allow construction of diverse units in the realms of
heterocyclic and natural product chemistry. Our research group has been studying various facets of this reaction, which include.
Search for new fast reacting electrophiles
Our group has interest in identification of fast reacting heterocyclic electrophiles for this otherwise slow paced reaction. In this
regard we have earlier shown that 5- or 3-isoxazolecarbaldehydes undergo very fast MBH reaction whereas 4-isoxazolecarbaldehyde reacts
slowly (Synthesis 2000, JOC, 2002, Synthesis 2003, Synthesis 2004). Placing a EWG in the ring however enhanced the rate considerably
(Synthesis 2004). The attributes responsible for the observed behavior about the reactivity was studied via DFT calculations
(ChemPhysChem 2013). We have also studied the MBH reactions of different pyrazolecarbaldehydes and have demonstrated how the proximity
of the formyl group to the heteroatom affects its reactivity (Arkivoc 2007). Our group has also pioneered to disclose the MBH reaction
of 1-formyl-9H-β-carboline (EJOC 2009). We have also studied the MBH reaction of indole-2-carbaldehyde and the utility of the adducts
to prepare the indoe-annulated systems (Tetrahedron 2010).
Synthesis of novel synthetic intermediates
One of the major aims of our group is to unfold the synthetic applications of the MBH adducts and the intermediates
obtained thereof. In this regard, our group has reported practical synthesis of primary allylamines which is scalable
(Synlett 2005, EJOC 2009). These allylamines were transformed into isonitriles (Synthesis 2009, Tetrahedron Lett. 2010)
which fortunately are colorless and odorless and can be employed in different IMCR for preparing useful heterocyclic
compounds. Exploring the utility of these allylamines for preparing chemical libraries of novel heterocycles is being a
continuing objective of this lab.
Synthesis of heterocycles
Aza-systems-Our research group has reported synthesis of a variety of aza-systems using MBH adducts or their
derivatives. This include even the annulated systems. Of special mention are aza-heterocycles such as quinolines,
quinazoline, pyridines, fused-pyrimidines, triazoles, tetrazoles, indoles, carbolines, 1,3- thiazines,
thiazolidinones.
Drug scaffolds-Our group has employed the MBH reaction as the key step for the formal synthesis of Tamiflu and total synthesis of Gabaculine a neurotransmitter (EJOC 2013).
Natural products and natural product mimics→ The MBH chemistry has been utilized by our group for the synthesis of a variety of β-carboline-based natural products or their mimics. This include canthinone, fascaplysin, arborescidine, maxonine and canthine. We have also developed synthetic strategies for accessing libraries of different β-carboline-based natural products. Additionally, the 1-formyl-9H-β-carboline is being used for template-directed synthesis of fused- β-carboline-based novel derivatives.
Transition-metal-based reactions
The advancement made in transition-metal-catalyzed reactions over the last 40 years has revolutionized the ways in which carbon-carbon (C-C) as well as carbon-heteroatom (CX) bonds are formed. As a result, there has been remarkable improvements in the protocols for the synthesis of natural products, pharmaceuticals, polymers and organic materials. Such coupling reactions are extremely useful for dissecting complex molecules in smaller subunits. This allows synthesis of highly diverse structural framework, which find use in identification of novel compounds for biological activity and the development of SAR. These reactions have also helped in the developing fine chemicals and useful drug intermediates. Moreover, the C-C and C-X coupling reactions have become effective tools to develop environmentally benign and economically sound manufacturing processes in a shorter period of time. Although there are diverse classes of reactions that are effected by different transition metals, our group is interested in-
Decarboxylative coupling-
The traditional cross-coupling reactions have certain limitations, which have been addressed in a variety of ways over the period. For
example, for the formation of biaryl, a metalleted arene intermediate is required for a reaction with a haloarene and it has to be
prepared separately. This limits the synthetic utility of the reaction as only limited numbers of such intermediates could be prepared.
However, several approximations have been attempted to overcome such limitation. Amongst them, one of the ways has been the use of
transition-metal-catalyzed decarboxylative coupling process. The interest of our group relies in the development of tandem protocol
involving decarboxylative coupling as the key step for preparing bioactive heterocycles and introducing knowledge based pharmacophore
via decarboxylative coupling leading to systems, which may find use as pharmaceuticals or for agriculture. In this context, we have
reported report a two component tandem protocol that combines a functional group transformation and an intramolecular decarboxylative
cross-coupling reaction which resulted into one-step synthesis of phenanthridines (CEJ 2013). We extended the study to the heterocyclic
substrates where we demonstrated the synthesis of a variety of fused-pyridine systems. It was observed that whereas the intramolecular
cross-couplings in pyrazole-based substrates proceeded smoothly in the presence of a [Pd-Cu] co-catalyst system, identical reactions in
thiophene-based substrates were successful in the presence of Pd-catalyst only (Org. Lett. 2013). Simultaneously we have also
accomplished the synthesis of N-substituted pyrroles and N-substituted azacyclic-2-carboxamide via tandem process wherein the
decarboxylation of the -amino acid leads to formation of a ylide which undergo further transformation (JOC 2015).
Oxidative C-H activation
The transition-metal oxidative coupling reactions have become a versatile tool for preparing variety of organic
compounds. The approach is considered to be attractive as it allows the coupling of two nucleophiles even. We
have been interested in the ligand-assisted ortho-C(sp2)-H activation of the phenyl ring for C-H functionalization.
In this regard we have demonstrated Palladium catalyzed pyrazole-directed regioselective oxidative
C(sp2)-H-functionalization of the N-phenyl ring in N-phenylpyrazoles to afford either a biaryl
bis-pyrazole (via dehydrogenative homocoupling) or N-(ortho-hydroxyphenyl)pyrazole (via C-H oxygenation)
or their mixture (JOC 2015). We have also demonstrated the palladium-catalysed alkoxylation in aryl
(β-carbolin-1-yl) methanones via β-carboline directed ortho-C(sp2)-H activation of aryl ring (OBC 2015).
We have been also interested in use of Iron nitrate (Fe(NO3)3.9H2O) for oxidative reactions. In this context,
we have developed iron nitrate mediated transformation of alkyne to nitrile (Adv. Synth. Catal. 2014).
We have also employed this reagent to develop oxidative Ugi reaction using benzyl amines as the aldehyde
surrogate (EJOC 2015) and oxidative Passerini reaction using primary alcohols as the aldehyde surrogate
(Arkivoc 2016).
Iodine-mediated cascade reactions
In recent times, organic transformations catalyzed by molecular iodine have attracted considerable attention.
A mild Lewis acid and electrophilic in nature, molecular iodine is inexpensive, readily available and non-toxic.
It has been successfully employed in reactions involving activation of π-system which proceeds either through
a charge transfer complex or via an iodoiranium /iodoirenium intermediate followed by attack of a nucleophile
either in an endo or exo fashion leading to the preparation of a variety of heterocycles. Such iodine-catalyzed
reactions not only complement several metal-catalyzed processes but are also considered environmentally benign.
We have been interested in iodine-catalyzed reactions since we discovered that the primary allylamines are
transformed to fused-pyridines including quinolines, pyrazolo[4,3-b]pyridines and thieno[3,2-b]pyridines via
Iodine-mediated intramolecular electrophilic aromatic cyclization (Org. Lett. 2012). Subsequently the approach
was extended for formulating the synthesis of dihydropyrazolo[4,3-c]azepines from suitably substituted
pyrazole-based allylamines via hydrative cyclization (Tetrahedron Lett. 2014). We have also developed
iodine-mediated electrophilic tandem cyclization of substituted 2-alkynylbenzaldehydes with anthranilic
acids under basic medium leading to iodo-1,2-dihydroisoquinoline-fused benzoxazinones (Tetrahedron 2013).
Recently, we developed NaNO2/iodine-mediated efficient one-pot transformation of Morita-Baylis-Hillman
(MBH) acetates into alkyl 3-nitro-5-(aryl/alkyl)isoxazole-4-carboxylates. The cascade event involved initial
Michael addition of NaNO2 onto the MBH acetate furnishes the allylnitro intermediate that undergoes I2-catalyzed
oxidative -C-H nitration of the nitromethyl subunit followed by [3+2] cycloaddition (Angew Chem. 2015).