Microbial infection research in CDRI focus on Tuberculosis, Fungal and Viral infection
Tuberculosis continues to kill millions of people worldwide (WHO Global Report, 2006) despite the disease is curable with a highly effective drug
regimen in the market. The failure of the present therapy stems largely from the lengthy course of the drugs-in-combination leading to poor compliance of the drugs.
This allows the emergence of multi drug resistance (MDR)-TB, the most urgent problem at present. Therefore, simplifying and shortening treatment for drug-sensitive
tuberculosis and providing new treatments for MDR-TB constitute two major goals in the development of novel drugs for Tuberculosis. TB research and drug screening
program at CDRI focus towards this end. Our team comprises biologists, chemists and pharmaceuticist who work synergistically in the different areas of TB drug
development research.
Tuberculosis Research
Drug Deign & Synthesis
➤Rational Drug Design based on virtual screening, de novo design, pharmacophore perception, SAR and ADME, Molecular docking, Conformational Analysis, Binding free energy simulations, Protein Ligand interactions etc.
➤ Asymmetric Synthesis of Trisubstituted Methanes as Anti-tubercular agents.
➤ Anti-tubercular agents based on sugar analogues and natural products.
➤ Structure-Function analysis of naturally occurring antimicrobial peptides and design of their novel analogs with higher therapeutic potential.
➤ Design of totally novel peptides with cell-selective activity and investigation on their biological activity against different microorganisms.
Screening & Drug Development
➤ In vitro, in vivo, macrophage based and high throughput screening. Lux-based assay system and BACTEC in use for screening of anti-TB compounds.
➤ Screening against selected targets and drug resistant non-pathogenic strain. Studies of PK/PD of anti-mycobacterial compounds.
➤ Screening for potent anti-bacterial compounds and evaluating their in-vivo efficacy.
➤ Drug repurposing for ESKAPE pathogens.
➤ The quality control and formulation development of anti-tubercular & antimicrobial candidates for drug development.
➤ Preparation and characterization of pulmonary delivery systems containing the anti-tubercular agents.
Significant Achievement
➤ Double recombinant BCG strain carrying firefly and Renila luciferase genes as two reporters has been generated to screen both primary and rationally synthesized antimycobacterial compounds.
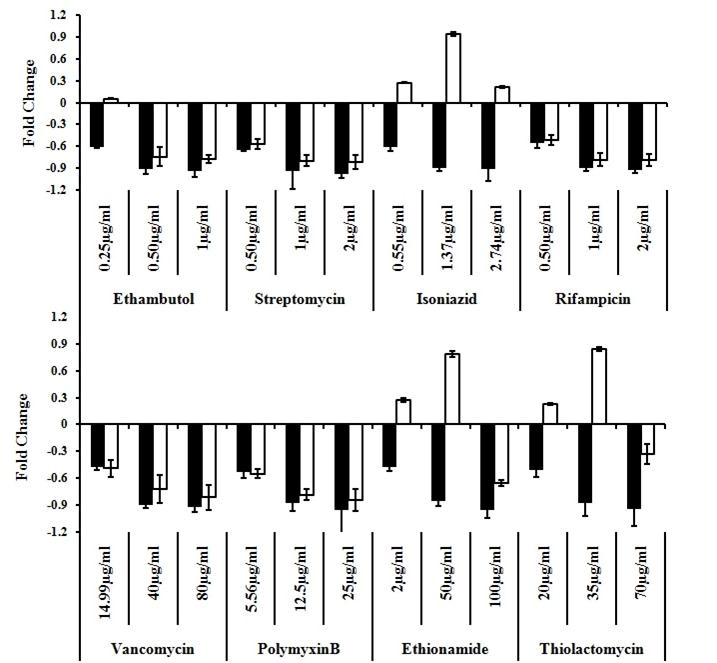
Note the reduced Fluc level (black bar) in response to all the drug treatments, while an enhanced Rluc level (blank bar) is seen in response to isoniazid, ethionamide, and thiolactomycin, which are known FAS-II pathway inhibitors.
➤ Development of murine infection model for persistence and reactivation.
➤ Target based screen systems for the screening of antimycobacterial drugs – against M.tb kinases (PknK and PknG), FASII elongation pathway, M.tb DNA ligase, malate synthase and ATP synthase.
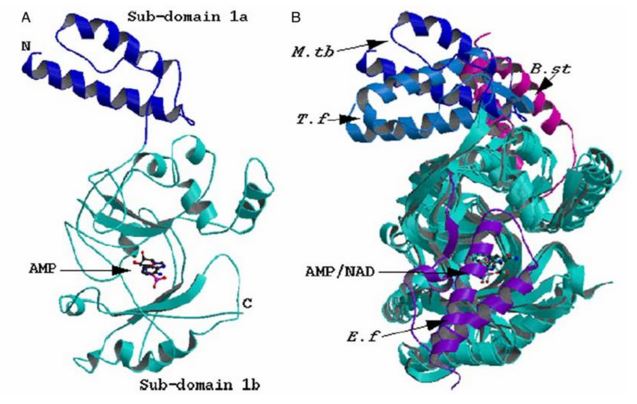
➤ Resolved the structure of adenylation domain of M. tuberculosis NAD+ dependent DNA ligase - a potential molecule for novel drug. LigA is a ~70 kD multi-domain enzyme which seals nicks in dsDNA. The enzyme is recognized as a novel target as it is found exclusively in bacteria and some viral sources.
➤ The first solution structure of Mycobacterium tuberculosis Pth protein (MtPth) using NMR- enables designing of novel inhibitors.
➤ Developed nano-sized delivery system of CDRI anti-TB molecules.
Fungal Infection
In vitro and in vivo evaluation of antifungal and antibacterial compounds/extracts and development of monoclonal antibodies for therapy and diagnosis.
Viral Infection
In vitro screening of anti-HIV (anti-RT) compounds and in vivo (mice) screening model for anti-Dengue virus compounds – Dr. R K Tripathi.
Recent Publications
➡ Verma SC, Agarwal P, Krishnan MY. Primary mouse lung fibroblasts help macrophages to tackle M. tuberculosis more efficiently and differentiate into myofibroblasts up on bacterial stimulation. Tuberculosis (Edinb). 2016, 97:172-80, PMID: 26586648 ➡ Agarwal P, Pandey P, Sarkar J, Krishnan MY. M. tuberculosis can gain access to adipose depots of mice infected via the intra-nasal route and to lungs of mice with an infected subcutaneous fat implant. Microb Pathog 2016, 93:32-7.
➡ Rishabh Sharma, Deepa Keshari, Kumar Sachin Singh, Shailendra Yadav & Sudheer K Singh. MRA_1571 is required for isoleucine biosynthesis and improves M. tuberculosis H37Ra survival under stress. Scientific Reports 2016, 6:27997.
➡ Singh KS and Singh SK. The M. Tuberculosis H37Ra gene Mra_1916 causes growth defects upon down-Regulation. Scientific Reports 2015, 5, 16131.
➡ Singh KP, Saxena R, Tiwari S, Singh DK, Singh SK, Kumari R and Srivastava KK. Rd-1 encoded espj protein gets phosphorylated prior to affect the growth and intracellular survival of mycobacteria. Scientific Reports 2015, 5, 12717
➡ Chatterjee A, Pandey S, Singh PK, Pathak NP, Rai N, Ramachandran R, Tripathi RP and Srivastava KK. Biochemical and functional characterizations of tyrosine phosphatases from pathogenic and nonpathogenic mycobacteria: indication of phenyl cyclopropyl methyl-/phenyl butenyl azoles as tyrosine phosphatase inhibitors. Applied Microbiology and Biotechnology 2015, 99(18), 7539-48
➡ Mishra NN, Ali S and Shukla PK. A monoclonal antibody against 47.2 kda cell surface antigen prevents adherence and affects biofilm formation of candida albicans. World Journal of Microbiology & Biotechnology 2015, 31(1), 11-21.
➡ Kumar, A and Shukla PK. A monoclonal antibody against glycoproteins of aspergillus fumigatus shows anti-adhesive potential. Microbial Pathogenesis 2015, 79, 24-30.
➡ Singh AK, Dutta D, Singh V, Srivastava V, Biswas RK and Singh BN. Characterization of M. smegmatis sigF mutant and its regulon: overexpression of SigF antagonist (MSMEG_1803) in M. smegmatis mimics sigF mutant phenotype, loss of pigmentation, and sensitivity to oxidative stress. Microbiologyopen 2015, 4(6), 896-916.
➡ Sashidhara KV, Rao KB, Kushwaha P, Modukuri RK, Singh P, Soni I, Shukla PK, Chopra S and Pasupuleti M. Novel chalcone-thiazole hybrids as potent inhibitors of drug resistant staphylococcus aureus. ACS Medicinal Chemistry Letters 2015, 6(7), 809-813
➡ Singh, Vandana; Biswas, Rajesh Kumar; Singh, Bhupendra N. Double recombinant M. bovis BCG strain for screening of primary and rationale-based antimycobacterial compounds. Ant. Agents and Chemotherapy 2014, 58(3), 1389 – 1396.
➡ Muthukumaresan T, Roy A, Suma S, Arockiaraj J and Pasupuleti M. A novel simple robust enzyme based high throughput screening method for antibacterial peptides discovery. Journal of Peptide Science 2014, 20(5), 341-348
➡ Singh SK, Tripathi DK, Singh PK, Sharma S and Srivastava KK. Protective and survival efficacies of Rv0160c protein in murine model of M. tuberculosis. Appl Microbiol Biotech 2013, 97 (13) 5825-5837,
➡ Biswas, RK, Dutta, D, Tripathi, A, Feng, YJ, Banerjee, M, Singh, BN. Identification and characterization of Rv0494: a fatty acid-responsive protein of the GntR/FadR family from M. tuberculosis. Microbiology-SGM (2013), 159: 913-923.
➡ Anirudh K Singh and Bhupendra N Singh. Differential expression of sigH paralogs during growth and different stress conditions in M. smegmatis. Journal of Bacteriology (2009): 191(8), 2888-93.
➡ Verma RK, Kaur J, Kumar K, Yadav AB, Misra A. Intracellular time course, pharmacokinetics, and biodistribution of isoniazid and rifabutin following pulmonary delivery of inhalable microparticles to mice. Ant. Agt. Chem. (2008): 52(9), 3195-201.
➡ Luthra A, Mahmood, A, Arora A and Ravishankar Ramachandran. Characterization of Rv3868: an essential hypothetical protein of the ESX-1 secretion system in M. tuberculosis J. Biol. Chem. (2008): 283, 36532-41.
➡ Pulavarti SV, Jain A, Pathak PP, Mahmood A, Arora A. Solution structure and dynamics of peptidyl-tRNAhydrolase from M. tuberculosis H37Rv. J Mol Biol. (2008): 18;378(1), 165-77.