Introduction
The Pharmaceutics group is mainly involved in analytical method development, quality assurance and pre-formulation studies on candidate drugs, and development of drug
delivery systems. Industry-academia collaborations are also undertaken for innovative product development research in the areas of biodegradable and biocompatible
nanoparticles and microparticles, lipid and surfactant vesicles, layer-by-layer (LBL) nano-fabrication, pulmonary delivery, transdermal delivery and conventional
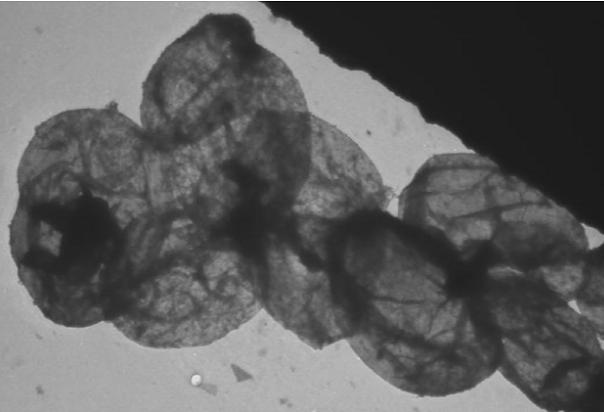
dosage forms. Current interests include delivery systems for infectious diseases, diabetes and osteoporosis. The transmission electron micrograph below shows hollow LBL
nanocapsules prepared in the lab.
Centchroman is one of CDRI’s Flagship drugs, currently under production at Hindustan Latex Ltd. The Pharmaceutics Division is currently engaged in generating data
required for product registration in other countries, and in development of novel formulations using this agent. We have recently licensed Consap Cream, a vaginal
application for contraception to HLL Lifecare. Alternative delivery systems for the active ingredients of Consap (Sapindus saponin) are under development.
A surprisingly efficacious herbal-based formulation against cerebral ischemia, code-named “Herbal Medicament” has also been licensed to Themis Medicare.
Collaboration for developing a novel dry powder inhalation formulation for use in tuberculosis is underway with M/s. Lupin Laboratories, funded by the NMITLI
program of CSIR. Links have also been established with the CSIR’s OSDD Initiative to investigate genome-wide transcription responses to drug delivery systems
prepared in the lab. Capacity-building in preparation of drug delivery systems using supercritical fluid methods is underway through a CSIR-Royal Society
collaboration with the Institute of Pharmaceutical Innovation, University of Bradford, UK. Work on nanometer-sized drug delivery systems has been pursued
fruitfully in collaboration with the Freie Universität, Berlin.
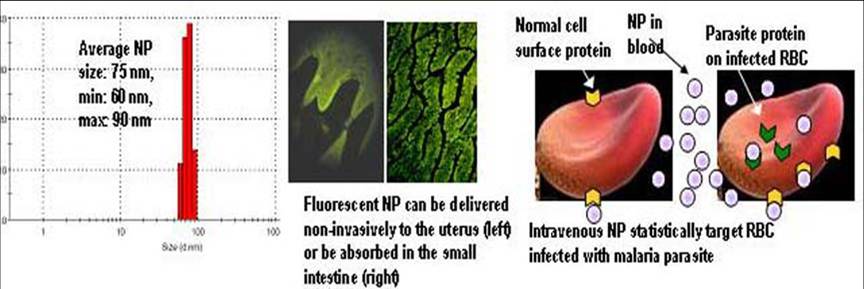
The bar chart below demonstrates our ability to prepare monodisperse, non-aggregating, reproducible batches of polymeric biodegradable nanoparticles of mean
diameter ~75 nm. Any of several possible known and candidate drugs, peptides, antigens and nucleic acids can be incorporated in these. The accompanying
fluorescent micrographs shows that such small particles instilled into the vagina of mice can overcome the barrier imposed by the cervix, and light up the walls
of the uterine endometrium. Administered orally, they can be taken up into lacteals of the intestinal villi. Nanoparticles targeting proteins expressed at higher
density on the surface of erythrocytes infected with the malaria parasite are under development.