Plasmodium parasite has evolved intricate mechanisms to avoid the development of TLR-mediated effector immune
responses. Toll-interacting protein (Tollip), a negative regulator of TLR-signaling plays a prominent role in pathogenesis in visceral
leishmaniasis; though, its role during Plasmodium infection has yet not been explored. We investigated the role of Tollip in Plasmodium
yoelii MDR (1×106) infected Balb/c mice on days 3-7 post-infection (having 5-40 % blood parasitemia, respectively). In our results, we have
witnessed that the level of Tollip increases at early time points and then goes down and again flares up at later stage when parasitemia
was 40% (Fig. 4). This data suggests the dual role of Tollip during early and late malaria infection. During early infection, Tollip regulates
excess TLR activation and might help in parasite multiplication. However, at later stage, when pro-inflammatory cytokines up-regulate,
Tollip again flares up to control the high inflammation and might play host-protective role. Further, the effect of Tollip expression on
cytokine response, parasitemia level and host survival is being validated using Tollip-knockdown mice.
Fig. Expression status of Tollip at different parasitemia
Various existing drugs (anticancer drugs, immunomodulator and antibiotics) were evaluated for their in vitro activity against human malaria parasite Plasmodium falciparum 3D7 (chloroquine-sensitive strain) and K1 (chloroquine-resistant strain). The IC50 values of selected drugs are reported in the fig. 5. Amongst all tested drugs, 3 drugs (5-Florouracil, Imiquimod and Moxifloxacin) from each group were selected for further screening in in vivo system on the basis of their promising in vitro activity. In Plasmodium yoelii infected swiss mice, these three drugs showed approximately 82-97% parasitemia suppression along with increased host mean survival time as compared to untreated group (fig. below).
1.3 Filariasis
1.3.1 Immunobiology of Lymphatic Filariasis: Immunogenicity and protective efficacy of recombinase-A protein of Wolbachia, an endosymbiont of
filarial nematode Brugia malayi.
Filarial parasites cause global morbidity. Wolbachia, an endo-symbiotic intracellular bacterium of the filarial nematode helps in their growth and
development, regulates fecundity in female worms and contributes to the immunopathogenesis of the disease. It is believed that several genes and
proteins of Wolbachia are intricately involved in either suppression, diversion, or polarization of the host immune response. However, genes and
proteins of Wolbachia that may act as putative vaccine candidates are not known. We cloned recombinase-A protein of Wolbachia from Brugia malayi
(wBmRecA) and carried out its detailed biochemical and immunological characterization. Bioinformatics analysis, circular dichroism and
fluorescence spectral studies showed significant sequence and structural similarities between wBmRecA and RecA of other alpha-proteo- bacterial
species (Fig. ➡).
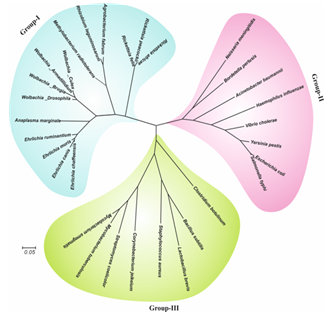
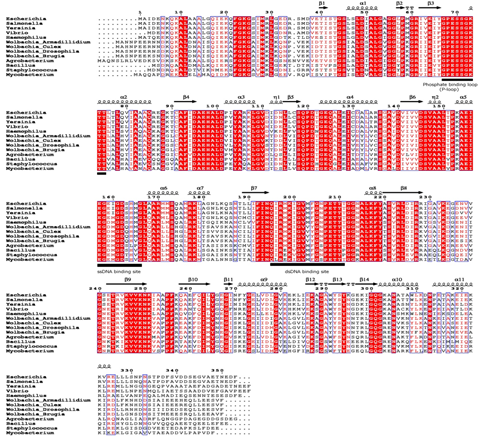
↖ Fig. Multiple sequence alignment of wBmRecA amino acid sequence with other RecA sequences was performed using CLUSTALW software. Identical and similar residues are denoted by red color background and boxed, respectively. The secondary structure is displayed onto the alignment. The amino acid residues involved in ATP binding (phosphate binding P-loop), single and double strand DNA binding are represented as bold underline. (B) Evolutionary relationship of wBmRecA among different bacterial species.
Notably, wBmRecA was ubiquitously expressed in all the three major life stages of B. malayi, including excretory-secretory products of the adult worm (Fig. 8 A-C).
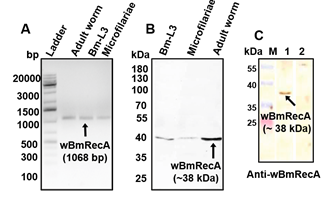
Fig. 8. Expression of wBmRecA in different life stages of B. malayi (A) RT-PCR analysis using cDNA from different stages of B. malayi. (B) Protein
lysate from different stages of B. malayi probed with anti-wBmRecA antibody. (C) Cross reactivity of anti- wBmRecA antibody with excretory-secretory
(ES) product from female worms (lane 1). No reactivity was observed in case of control sera (lane 2).
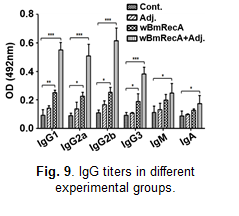
In silico studies suggested immunogenic potential of wBmRecA, and mice immunized with wBmRecA exhibited elevated levels of immunoglobulins IgG1, IgG2a,
IgG2b and IgG3 in their serum (Fig. 9) along with increased percentages of CD4+, CD8+ T cells and CD19+ B cells in their spleens.
Interestingly, splenocytes from immunized mice showed increased m-RNA expression of T-bet, elevated proinflammatory cytokines IFN-γ and IL-12, while
peritoneal MΦs exhibited increased levels of iNOS, downregulated Arg-1 and secreted copious amounts of nitric oxide which contributed to severely
impaired development of the third stage infective larvae (Bm-L3).
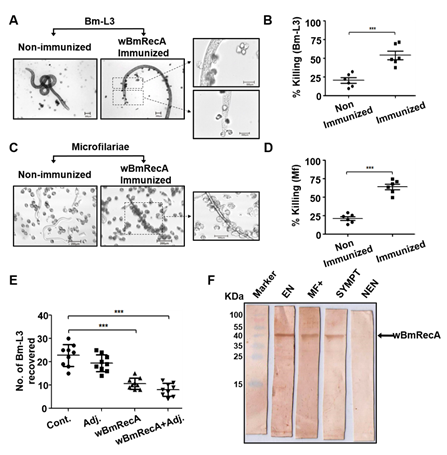
Fig. Host protective nature of wBmRecA. Peritoneal exudate cells from naive mice were separately cultured with (A) Bm-L3 and (C) Mf in the presence
or absence of sera from r-wBmRecA immunized animals and (B) Bm-L3 and (D) Mf killing was observed microscopically. (E) Recovery of Bm-L3 in mice
from different treatment groups. (F) Sero-reactivity of wBmRecA with human bancroftian serum samples. Lane 1- Protein molecular weight marker;
lane 2- serum from endemic normal (EN); lane 3- serum from asymptomatic microfilariaemic carriers (MF+); lane 3- serum from symptomatic
microfilariaemic (SYMPT) and lane 4- serum from non-endemic normal (NEN) controls.
Interestingly, sera from immunized mice promoted significant cellular adherence and cytotoxicity against microfilariae and Bm-L3 (Fig. 10 A-E) and
wBmRecA demonstrated strong immuno-reactivity with bancroftian sera from endemic normal individuals (Fig. 10F) which suggest that wBmRecA is highly
immunogenic, and should be explored further as a putative vaccine candidate against lymphatic filariasis (Vaccine, 2019 , 37(4),
571-580 https://doi.org/10.1016/j.vaccine.2018.12.015).
1.4 Medicinal Chemistry
1.4.1 Malaria
Aminopropanols
In continued effort to assess the antiplasmodial efficacy of the aminopropanols, 30 new analogues were synthesized and submitted for activity. The
synthesis involved the incorporation of heterocyclic scaffolds in the aminopropanol unit. These compounds displayed significant activity against 3D7
as well as K1 strains of P. falciparum. Encouraged by these results, synthesis of a novel class of diaminopropanol compounds by incorporating an
additional amino group in the molecule and their derivatives was accomplished. All 10 new compounds of this class, displayed enhanced bioactivity in
the range of IC50 0.04-0.250 µM for 3D7 CQS and 0.07-0.91 µM for K1 CQR strains of P. falciparum. Two compounds S-019-190 & S-019-0192 were
resynthesized for carrying out the in vivo studies. However, the 10 new compounds bearing nitropyridine and 3-aminoquinoline scaffolds were found to
eleicit poor biological response.

1-(Arylsulfonyl)pyrrolidine-2-carboxamides
During this period around 20 new 1-(substituted phenylsulfonyl)pyrrolidine-2-carboxamides were prepared and subjected for evaluation as antiplasmodial agent. However the compounds were found to be inactive against the 3D7 CQS strain of P. falciparum.
Heterocycles
Compounds belonging to various heterocycles including oxaboroles, triazoles, indolinone, spiroindolinones, pyrazoles, isoxazoles,
pyrrolo[1,2-a]isoquinolines, 4-aminoquinolines, phosphorylated naphthalenes were prepared and submitted for evaluation as antiplasmodial agents.
Of these a few analogues displayed antiplasmodial efficacy ranging between 2-4 M against 3D7 CQS strain of P. falciparum.
1.5 Anti-parasitic screening for drug discovery
1.5.1 Anti-malarial screening
633 compounds were screened against the human malaria parasite, P. falciparum (CQ-sensitive PF3D7 and CQ-resistant K1 strain). These compounds belong
to diverse chemical classes such as quinoline triazols, oxazenes, oxazole salt, di-aminopropanol, pyrimidine based hetrocyclic derivatives,
HDAC inhibitors, spiroindoles, peptidyl hydroxamic and dihydronaphthalene.
Quinoline triazol (20) derivatives (S019-0546, 0547, 0548, 0549, 0550, 0551, 0552, 0553, 0554, 0555, 0556, 0557, 0558, 0559, 0560, 0561, 0562, 0563,
0564 and 0565) had IC50 in the range of 0.03 to 0.35 µM against CQ-sensitive (PF3D7) and 0.03 to 1.91 µM against CQ-resistant strain (K1). Oxazene
class (10) compounds (S019-0472, 0473, 0474, 0475, 0476, 0477, 0478, 0479, 0510 and 0511) exhibited activity with IC50 in the range of 0.11 to 0.97
µM against PF3D7 and 0.07 to 0.48 µM against K1. Oxazole salt derivatives (23) (S019-0129, 0131, 0132, 0133, 0134, 0135, 0136, 0137, 0139, 0140,
0141, 0142, 0156, 0157, 0158, 0159, 0160, 0161, 0163, 0164, 0165, 0168, 0169) showed activity with IC50 values between 0.24 to 0.97 µM against 3D7
and 0.23 to 2.34 µM against K1 strain. Di-aminopropanols (10) compounds (S019-0117, 0118, 0119, 0120, 0182, 0184, 0187, 0190, 0192 and 0212)
exhibited antimalarial effect with IC50 values of 0.04 to 0.25 µM against PF3D7 and 0.24 to 1.96 µM against K1. Aminopropanol derivatives
(S018-0507, 0251 and 0509) exhibited IC50 ranging from 0.12 -0.78 µM against Pf3D7 and <0.07 - 2.94 µM against K1 strain. Compounds
(S019-0464, 0065 and 0067) belonging to pyrimidine class exhibited activity in the range of 0.17 and 0.46 µM (IC50 values) against Pf3D7and
0.24 to 0.61 µM against K1. Compound designed based on HDAC inhibitor (S019-0077) had IC50 values of 0.22 µM against 3D7 and 0.66 µM against
K1. Spiroindole derivatives (S018-0309 and S018-0312) showed IC50 of 0.88 and 0.48 µM against 3D7 and 1.47 and 3.11 µM against K1 strain. Peptidyl
hydroxamic acid (2) derivatives (S018-0396 and 0411) had IC50 values of 0.94 and 0.59 µM, respectively, PF3D7 strain and 0.81 and 0.54 µM,
respectively against K1 strain. Indole based scaffold, S019-0010 had IC50 values of 1.04 µM against 3D7 and 0.62 µM against K1. Dihydronaphthalene
derivatives (S018-0489, 0490 and S017-0670) exhibited IC50 values 0.97, 0.03 and 0.72µM respectively, against PF3D7 and 0.63, 0.02 and 3.99 µM
respectively, against K1. Furan based compound (S018-0485) exhibited IC50 at 0.86 µM against 3D7 and 0.91 µM against K1 strain. Imidazopyridine
class compound (S019-0019) showed IC50 at 0.60 µM against PF3D7 and 0.15 µM against K1 strain. S019-0124, S019-0200, S019-077 and S019-0608 showed
antimalarial activity in the range of 0.2-0.5 µM in CQ-sensitive and resistant strains. These compounds were evaluated for cytotoxic profile against
Vero cell line and were found to have minimal or no cytotoxicity.
1.5.2 In vitro antimalarial activity report of compounds received under DBT Twinning project during Jan 2019 – Dec 2019
During the reported period, 40 compounds were received under DBT Twining project and were screened against PF3D7 and K1 strains. Out of these, 10
compounds namely SKQ-1C, 1D, 1E, 2C, 2D, 2E, 3B, 3C, 3D and 3E have shown IC50 as 0.05, 0.04, 0.35, 0.14, 0.05, 0.04, 0.17, 0.14, 0.07 and 0.08
µM against Pf3D7 chloroquine sensitive and 0.99, 0.94, 0.31, 0.34, 0.79, 0.28, 0.38, 0.63, 0.22 and 0.29 µM against PfK1 chloroquine resistant
strain, respectively. These molecules were also evaluated for cytotoxic profile in Vero cell line and found to be safe.
1.5.3 In vitro antimalarial activity report of plant extracts received across the country (Jan 2019 – Dec 2019)
During the reporting period, two plant extracts received from other research institutes were screened against the P. falciparum. Both plant extracts,
namely RMRC-001 & RMRC-002 have shown antimalarial activity with their IC50 values of 27.45 and 19.65 µg/ml against CQ sensitive Pf3D7 strain and
5.10 & 36.85 µg/ml against CQ resistant PfK1 strain, respectively. These molecules are found to be safe in Vero cells.
1.5.4 In vivo antimalarial profile of CSIR-CDRI compounds against P. yoelii nigeriensis N67 (CQ resistant) (Jan 2019 – Dec 2019)
Four compounds, namely S018-0248 (aminopropanol class), S017-0383 (Nitropyridine class), S019-0065 and S019-0067 (pyrimidine based hetrocyclic class)
were administered for 4 days, at 100 mg/kg in swiss mice infected with P. yoelii nigeriensis N67 infected. Out of these, only S018-0248 showed 38.34%
parasite suppression on day 4 post treatment but was not curative on day 28. S018-0485 (furan derivatives), S018-0490 and S018-0489
(dihydronaphthalene derivatives) were dosed for 4 consecutive days, at 100 mg/kg which resulted in 18.87, 21.60 and 0%, respectively, parasite
suppression on day 4. S018-0248 (aminopropanol class) was also tested at the same dose and showed 38.34% parasite suppression on day 4. S017-0383
(nitropyridine) was dosed for 4 consecutive days (100 mg/kg) and it did not show any parasite suppression on day 4.
1.5.5 Anti-leishmanial screening
Novel synthetic moieties representing several prototypes viz., quinoline-imidazole hybrids, pyrolo quinolinone, chalcones, stilbenes, phenyl
allylindole, imidazo pyrolo quinolone derivatives, quinoline tetrahydropyrimidine etc. were synthesized and tested for their efficacy against
experimental model of visceral leishmaniasis. Total sixty seven synthetic compounds were evaluated at 25 μM and 12.5 μM concentrations respectively
against in vitro macrophage-amastigote model for lead identification. Compounds belonging to pyrimidine-based heterocyclic derivates
(S-018-0699, S-018-0701) series showed significant anti-amastigote activity (>80% inhibition of amastigote multiplication and SI >5). Half-maximal
inhibitory concentrations (IC50) of S-018-0699 and S-018-0701 were found to be 9.28 µM and 8.77 μM respectively against intracellular amastigotes.
S-018-0699 and S-018-0701 will be further evaluated for their anti-leishmanial activity in L. donovani- golden hamster model.
153 Novel synthetic moieties representing several prototypes viz., Isoxazide, Triazol, Nitropyridine, Aminothiophenol, Aminopyridine, Naphthol
Hetrocyclic, Isoxazole, Oxazoline, Benzofuran Nitropyridine, Oxazoline, Isoxazole, Isoxazide amide, Dihydroxy-hex-2-en-1-one, and 16 analogues of
lead compound 96/261 were screened in vitro against promastigotes and amastigotes. Out of these 27 compounds exhibited IC50 < 10µM and SI
index > 5.
Six compounds were evaluated for their in vivo efficacy in L. donovani / Hamster model at 50mg/Kg I.P or 100mg/Kg oral dose. Only one compound
(analogue of 96/261) showed >70% anti-leishmanial efficacy in L. donovani golden hamster model at 100mg/Kg oral dose for 5 days.